
The major product in the above-mentioned reaction is:
1.

2.

3.

4.






The alkane that gives only one monochloro product on chlorination with \(\text{Cl}_2\) in presence of diffused sunlight is-
| 1. | 2,2,-Dimethylbutane | 2. | Neopentane |
| 3. | n-Pentane | 4. | Isopentane |
In the following reaction,
\(\mathrm{ CH_{3}C\equiv CH\xrightarrow[Red \ hot \ iron \ tube]{873 K} \ A}\)
The number of () bonds present in the product (A) is:
| 1. | 21 | 2. | 9 |
| 3. | 24 | 4. | 18 |
2,2–dimethylpropane, C(CH3)4, is an isomer of pentane, CH3(CH2)3CH3. Pentane has a boiling point of 36 °C whilst the boiling point of 2,2–dimethylpropane is 10 °C.
The statement that explains the difference in the boiling points for these two substances is:
| 1. | The molecules have different relative molecular masses. |
| 2. | Isomers have different chemical properties. |
| 3. | Pentane has permanent dipoles. |
| 4. | Longer chain, lesser branched molecules have stronger spontaneous/induced dipoles. |
Which of the following would exhibit cis-trans isomerism?
1.
2.
3.
4.
In the following reaction, the number of ( σ ) bonds present in the product (A) is:
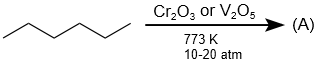
| 1. | 21 | 2. | 9 |
| 3. | 24 | 4. | 12 |
| 1. | |
| 2. | |
| 3. | |
| 4. |  |
The most suitable reagent for the following conversion is-
| 1. | Hg2+/ H+, H2O | 2. | Na/liquid NH3 |
| 3. | H2, Pd/C, quinoline | 4. | Zn/HCl |
An alkene "A" on reaction with and gives propanone and ethanal in equimolar ratio. The addition of HCl to alkene "A" gives "B" as the major product. The structure of product "B" is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
|
Given below is a reaction sequence: |
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |








