Which one of the following compounds of the formula does not decolorize bromine water?
1. But-1-ene
2. But-2-ene
3. Cyclobutane
4. 2-methyl propene
Consider the given reaction:
Compound x and y, respectively in the above reaction is:
| 1. | 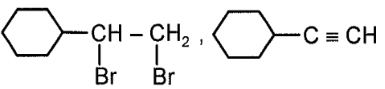 |
| 2. | 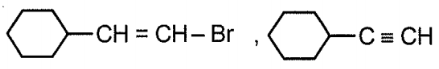 |
| 3. | 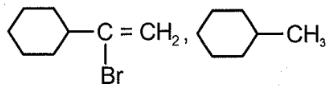 |
| 4. | 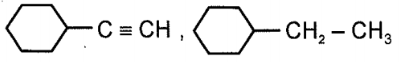 |
Match the reagent from Column I which on reaction with CH3-CH=CH2 gives the same product given in Column II as per the codes given below:
| Column I | Column II | ||
| A. | O3/Zn+H2O | 1. | Acetic acid and CO2 |
| B. | KMnO4/H+ | 2. | Propan-1-ol |
| C. | dil. KMnO4/OH- | 3. | Propane-1,2-diol |
| D. | B2H6/NaOH and H2O2 | 4. | Acetaldehyde and formaldehyde |
Codes:
| Options: | A | B | C | D |
| 1. | 4 | 1 | 3 | 2 |
| 2. | 1 | 2 | 3 | 4 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 1 | 2 | 3 |
Among the following reactions, the incomplete combustion of methane is:
| 1. | \(2 {CH}_4+{O}_2 \xrightarrow[]{\mathrm{Cu/523 \ K/100 \ atm}} 2 {CH}_3 {OH}\) |
| 2. | \({CH}_4+{O}_2 \xrightarrow[]{{Mo_{2}O_{3}}} {HCHO}+{H}_2 {O}\) |
| 3. | \(CH_4 + O_2 → C + 2H_2O\) |
| 4. | \(CH_4 + 2O_2 → CO_2 + 2H_2O\) |
| Assertion (A): | Eclipsed and staggered are two conformations of ethane. |
| Reason (R): | In all the conformations, the bond angles and bond lengths change. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
Which of the following is/are aromatic structure(s)?
| a. |  |
b. |  |
| c. |  |
d. |  |
| 1. | a and b | 2. | b and c |
| 3. | c and d | 4. | a and c |
2,2–dimethylpropane, C(CH3)4, is an isomer of pentane, CH3(CH2)3CH3. Pentane has a boiling point of 36 °C whilst the boiling point of 2,2–dimethylpropane is 10 °C.
The statement that explains the difference in the boiling points for these two substances is:
| 1. | The molecules have different relative molecular masses. |
| 2. | Isomers have different chemical properties. |
| 3. | Pentane has permanent dipoles. |
| 4. | Longer chain, lesser branched molecules have stronger spontaneous/induced dipoles. |
Mark the correct order of reactivity towards the electrophilic aromatic substitution reaction.
| 1. | I > II > III > IV | 2. | IV > III > II > I |
| 3. | II > I > IV > III | 4. | II > I > III > IV |
In the following reaction, the number of ( σ ) bonds present in the product (A) is:
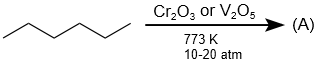
| 1. | 21 | 2. | 9 |
| 3. | 24 | 4. | 12 |
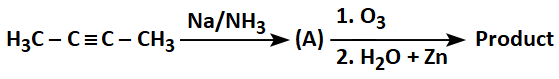
| 1. | 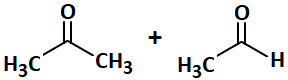 |
| 2. | 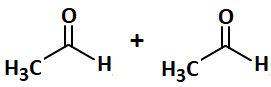 |
| 3. | 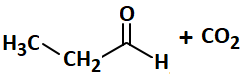 |
| 4. | 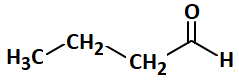 |








