Consider the given reaction:

Compound x and y, respectively in the above reaction is:
1.
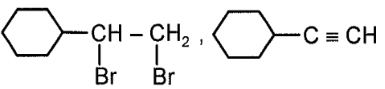
2.
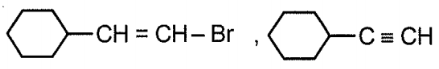
3.
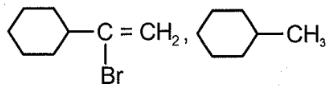
4.
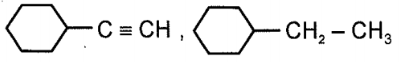

Compound x and y, respectively in the above reaction is:

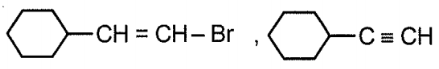
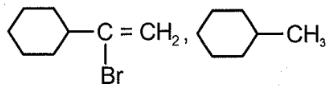
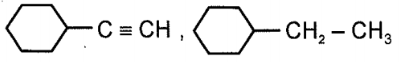
The major product (P), in the reaction given below is:

| 1. |  |
2. |  |
| 3. |  |
4. |  |
The major product (P) in the following reaction is:

| 1. |  |
2. |  |
| 3. |  |
4. |  |
Which one of the following compounds of the formula does not decolorize bromine water?
1. But-1-ene
2. But-2-ene
3. Cyclobutane
4. 2-methyl propene
Which of the following statement(s) is/are correct about the elimination reaction of 2-Bromopentane to form pent-2-ene:
(a) -Elimination reaction
(b) Follows Zaitsev rule
(c) Dehydrohalogenation reaction
(d) Dehydration reaction
| 1. | (a), (c), (d) | 2. | (b), (c), (d) |
| 3. | (a), (b), (d) | 4. | (a), (b), (c) |
An alkene on ozonolysis gives methanal as one of the products. Its structure is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
| 1. | |
| 2. | |
| 3. | |
| 4. |  |
In the following reaction, the number of ( σ ) bonds present in the product (A) is:

| 1. | 21 | 2. | 9 |
| 3. | 24 | 4. | 12 |
Which of the following would exhibit cis-trans isomerism?
1.
2.
3.
4.
2,2–dimethylpropane, C(CH3)4, is an isomer of pentane, CH3(CH2)3CH3. Pentane has a boiling point of 36 °C whilst the boiling point of 2,2–dimethylpropane is 10 °C.
The statement that explains the difference in the boiling points for these two substances is:
| 1. | The molecules have different relative molecular masses. |
| 2. | Isomers have different chemical properties. |
| 3. | Pentane has permanent dipoles. |
| 4. | Longer chain, lesser branched molecules have stronger spontaneous/induced dipoles. |






