Select Question Set:
In the given reaction, the major product is:

1.

2.

3.

4.





Subtopic: Ethers: Preparation & Properties, Uses |
83%
Level 1: 80%+
Hints
| I: | The product of the reaction of phenol with bromine depends on the nature of the solvent. |
| II: | The reaction of phenol with bromine in CHCl3 gives a monosubstituted bromo derivative whereas the reaction of phenol with bromine water yields a trisubstituted bromo derivative of phenol. |
In light of the above statements, choose the most appropriate answer from the options given below:
| 1. | I is correct and II is incorrect |
| 2. | I is incorrect and II is correct |
| 3. | Both I and II are correct |
| 4. | Both I and II are incorrect |
Subtopic: Electrophilic Substitution Reactions, Uses of Phenols |
85%
Level 1: 80%+
NEET - 2022
Hints
The correct order of acidic strength of the following molecules is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Subtopic: Alcohols: Preparation & Properties |
65%
Level 2: 60%+
NEET - 2022
Hints
Consider the following structures:
The resonating structures of phenol among the given structures above are:
1. II and III only
2. I, II and III only
3. I, II and IV only
4. II and IV only
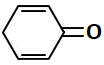 |
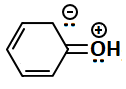 |
| I | II |
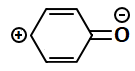 |
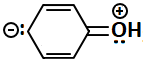 |
| III | IV |
The resonating structures of phenol among the given structures above are:
1. II and III only
2. I, II and III only
3. I, II and IV only
4. II and IV only
Subtopic: Electrophilic Substitution Reactions, Uses of Phenols |
73%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
The correct order of the solubility of different alcohols in water is:
1. Ethyl alcohol>𝑛-Propyl alcohol > 𝑛-Butyl alcohol
2. 𝑛-Propyl alcohol > Ethyl alcohol > 𝑛-Butyl alcohol
3. Ethyl alcohol > 𝑛-Butyl alcohol > 𝑛-Propyl alcohol
4. 𝑛-Butyl alcohol > 𝑛-Propyl alcohol > Ethyl alcohol
Subtopic: Alcohols: Preparation & Properties |
80%
Level 1: 80%+
Please attempt this question first.
Hints
Please attempt this question first.
C4H10O gives white precipitate within 5 min with concentrated hydrochloric acid in the presence of anhydrous zinc chloride.
The alcohol can be:
| 1. | 2. |  |
|
| 3. | 4. |  |
Subtopic: Mechanism of Dehydration, Methanol & Ethanol |
76%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
The correct structure of 2-Bromo-4-methyl anisole is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Ethers: Preparation & Properties, Uses |
91%
Level 1: 80%+
Please attempt this question first.
Hints
Please attempt this question first.
The structural isomers C2H5OH and CH3OCH3 should be anticipated to have the same values for which of the following parameters?
(Assume ideal behaviour):
| 1. | Heat of vaporisation |
| 2. | Vapour pressure at the same temperature |
| 3. | Boiling point |
| 4. | Gaseous densities at the same temperature and pressure |
Subtopic: Ethers: Preparation & Properties, Uses |
70%
Level 2: 60%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.
The number of isomeric alcohols (excluding stereoisomers) of molecular formula C6H14O which give a positive iodoform test are:
1. Three
2. Four
3. Five
4. Two
1. Three
2. Four
3. Five
4. Two
Subtopic: Alcohols: Preparation & Properties |
Level 3: 35%-60%
NEET - 2013
Hints
Consider the following reaction and identify the product (P).


| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Alcohols: Preparation & Properties |
72%
Level 2: 60%+
NEET - 2023
Hints
Select Question Set:






