Decreasing order of acidic behavior of benzene, n-hexane, and ethyne is:
1. Hexane > Ethyne > Benzene
2. Benzene > Hexane > Ethyne
3. Ethyne > Benzene > Hexane
4. Benzene > Ethyne > Hexene
The correct order of decreasing reactivity of the following compounds:
Chlorobenzene, 2,4-Dinitrochlorobenzene, and p-Nitrochlorobenzene
with an electrophile (E+) is :
| 1. | Chlorobenzene > p–Nitrochlorobenzene > 2,4-Dinitrochlorobenzene |
| 2. | p – Nitro chlorobenzene > 2, 4-Dinitrochlorobenzene > Chlorobenzene |
| 3. | Chlorobenzene > 2, 4-Dinitrochlorobenzene > p-Nitrochlorobenzene |
| 4. | 2, 4-Dinitrochlorobenzene > Chlorobenzene > p-Nitrochlorobenzene |
Match the reagent from Column I which on reaction with CH3-CH=CH2 gives the same product given in Column II as per the codes given below
| Column I | Column II | ||
| A. | O3/Zn+H2O | 1. | Acetic acid and CO2 |
| B. | KMnO4/H+ | 2. | Propan-1-ol |
| C. | dil. KMnO4/OH- | 3. | Propane-1,2-diol |
| D. | B2H6/NaOH+ and H2O2 | 4. | Acetaldehyde and formaldehyde |
Codes:
| Options: | A | B | C | D |
| 1. | 4 | 1 | 3 | 2 |
| 2. | 1 | 2 | 3 | 4 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 1 | 2 | 3 |
Arrange the following alkyl halides in decreasing order of the rate of -elimination reaction with alcoholic KOH.
| A. | 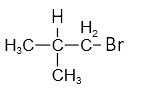 |
| B. | CH3-CH2-Br |
| C. | CH3-CH2-CH2-Br |
1. A > B > C
2. C > B > A
3. B > C > A
4. A > C > B
Among the following reactions, the incomplete combustion of methane is:
| 1. | \(2 {CH}_4+{O}_2 \xrightarrow[]{\mathrm{Cu/523 \ K/100 \ atm}} 2 {CH}_3 {OH}\) |
| 2. | \({CH}_4+{O}_2 \xrightarrow[]{{Mo_{2}O_{3}}} {HCHO}+{H}_2 {O}\) |
| 3. | \(CH_4 + O_2 → C + 2H_2O\) |
| 4. | \(CH_4 + 2O_2 → CO_2 + 2H_2O\) |
The products formed after ozonolysis of Pent-2-ene are:
| 1. | Ethanal and Methanal | 2. | Ethanal and Propanal |
| 3. | Ethanal and Butanal | 4. | Ethanal and Ethanol |
Which of the following is a free radical substitution reaction?
1. Benzene with Br2/AlCl3
2. Acetylene with HBr
3. Methane with Br2/hv
4. Propene with HBr/(C6H5COO)2
Mark the correct order of reactivity towards the electrophilic aromatic substitution reaction.
| 1. | I > II > III > IV | 2. | IV > III > II > I |
| 3. | II > I > IV > III | 4. | II > I > III > IV |
Which of the following is/are aromatic structure(s)?
| a. |  |
b. |  |
| c. |  |
d. |  |
| 1. | a and b | 2. | b and c |
| 3. | c and d | 4. | a and c |
An alkene on ozonolysis gives methanal as one of the products. Its structure is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |







