The set of reagents used to produce freon (CCl2F2) are:
1.
C + F2 + Cl2 →
2.
CH3Cl + F2 →
3.
\(CCl_4 + HF \ \xrightarrow[]{SbCl_5} \)
4.
CCl4 + F2 →
\(HC\equiv CNa \ + \ Cl-CH_{2}-CH_{2}-CH_{2}-I \ \rightarrow \ (A) \)
The major product (A) in the above reaction is :
1. H-CC-CH2-CH2-CH2-I
2. CH2=CH-CH2-I
3. H-CC-CH2-CH2-CH2-Cl
4. CH2=CH-CH2-Cl
The product (C) in the below-mentioned reaction is:
\(CH_{3}CH_{2}CH_{2}Br \ \xrightarrow[]{\textbf{KOH(alc.)}} \)
\(\ (A) \ \xrightarrow[]{\textbf{HBr}} \ (B) \ \xrightarrow[]{\textbf{KOH(aq}} \ (C)\)
| 1. | Propene | 2. | Propyne |
| 3. | Propan-1-ol | 4. | Propan-2-ol |
The correct sequence of the dipole moment of given alkyl halides is :
1. CH3F > CH3Cl > CH3Br > CH3I
2. CH3F < CH3Cl < CH3Br < CH3I
3. CH3Cl > CH3F > CH3Br > CH3I
4. CH3Cl > CH3Br > CH3F > CH3I
Select the correct order of boiling points of the given alkyl halides
i. n-Butyl chloride
ii. iso-Butyl chloride
iii. Tertiary butyl chloride
iv. Secondary butyl chloride
1. i > ii > iii > iv
2. i > iii > ii > iv
3. i > ii > iv > iii
4. i > iv > ii > iii
The products (A) and (B) in the below reactions are, respectively:
3CH3Cl + SbF3 → 3CH3F + SbCl3
The name of the above reaction is:
1. Finkelstein reaction.
2. Sandmeyer's reaction.
3. Gattermann reaction.
4. Swart's reaction.
An reaction at an asymmetric carbon of a compound always gives
1. An enantiomer of the substrate
2. A product with opposite optical rotation
3. A mixture of diastereomers
4. A maximum racemized product
The most reactive compound for SN2 reaction among the following is:
| 1. | 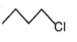 |
2. | 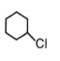 |
| 3. | 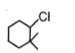 |
4. | 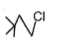 |
| Assertion (A): | The reactivity of alkyl halide for nucleophilic substitution reaction follows the order Rl > RBr > RCl |
| Reason (R): | Leaving ability of halide ions follows the order: I- > Br- > Cl- |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |







