A Carnot engine has an efficiency of \(50\%\) when its source is at a temperature \(327^\circ \mathrm{C}\). The temperature of the sink is:
1. \(200^\circ \mathrm{C}\)
2. \(27^\circ \mathrm{C}\)
3. \(15^\circ \mathrm{C}\)
4. \(100^\circ \mathrm{C}\)

| 1. | \(200\) J | 2. | zero |
| 3. | \(400\) J | 4. | \(600\) J |
An ideal gas undergoes a thermodynamic process described by the equation:
\(PV^2=C,\)
where \(C\) is a constant. The gas transitions from an initial state \((P_1, V_1, T_1)\) to a final state \((P_2, V_2, T_2).\) Which of the following statements is correct?
| 1. | \(\text{If}~P_1>P_2,~\text{then}~T_1<T_2 \) |
| 2. | \(\text{If}~V_2>V_1,~\text{then}~T_2>T_1\) |
| 3. | \(\text{If}~V_2>V_1,~\text{then}~T_2<T_1\) |
| 4. | \(\text{If}~P_1>P_2,~\text{then}~V_1>V_2\) |
A gas undergoes an isothermal process. The specific heat capacity of the gas in the process is:
| 1. | infinity | 2. | \(0.5\) |
| 3. | zero | 4. | \(1\) |

| 1. | \(4\) | 2. | \(1\) |
| 3. | \(2\) | 4. | \(3\) |

| 1. | \(30~\text J\) | 2. | \(-90~\text J\) |
| 3. | \(-60~\text J\) | 4. | zero |
1. \(P^{\gamma-1} {~T}^\gamma \)
2. \(T V^{1-\gamma} \)
3. \(V^{\gamma-1} {~T}^ \gamma \)
4. \(P^{1-\gamma} ~{T}^\gamma\)
The graph below shows the processes involved in a Carnot engine.
For the two isotherms 1 and 2, the temperature of isotherm 1 is:
1. Equal to the temperature of isotherm 2
2. More than the temperature of isotherm 2
3. Less than the temperature of isotherm 2
4. Sometimes more & sometimes less than the temperature of isotherm 2
The graph given below shows variation of specific heat capacity of water versus temperature.
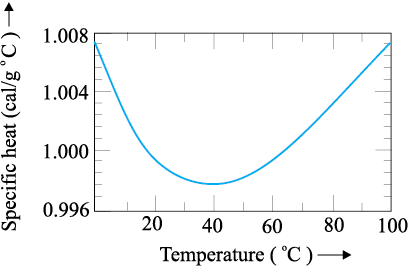
From the graph given above, we can conclude that:
1. Specific heat capacity always increases
2. Specific heat capacity always decreases
3. Specific heat capacity never changes
4. Specific heat capacity first decreases and then increases
The \((P\text{-}V)\) diagram for an ideal gas in a piston-cylinder assembly undergoing a thermodynamic process is shown in the figure. The process is:
| 1. | adiabatic | 2. | isochoric |
| 3. | isobaric | 4. | isothermal |








