Polyamide is represented by:
1. Teflon
2. Nylon – 6,6
3. Terylene
4. Bakelite
The polymer that is used as a substitute for wool in making commercial fibres is -
1. Melamine
2. Nylon-6, 6
3. Polyacrylonitrile
4. Buna-N
The structure of the monomer used for the preparation of plexiglass is
1. ![]()
2. 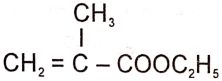
3. 
4. 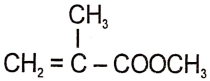
Select the correct option based on statements below:
| Assertion (A): | Vulcanisation of rubber is heating of synthetic rubber with sulfur. |
| Reason (R): | Stiffness is lost in vulcanization. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
Plastic household crockery is prepared by using :
1. Melamine and tetrafluoroethane.
2. Malonic acid and hexamethylene imine.
3. Melamine and vinyl acetate.
4. Melamine and formaldehyde.
The polymer that is addition as well as copolymer is:
1. PHBV
2. PVC
3. Buna-S
4. Neoprene
Match the polymers given in Column-I with their repeating units given in Column-II. Choose the correct option from the codes given below :
| Column-I | Column-II |
| (a) Polystyrene | (p) 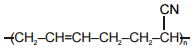 |
| (b) Novolac | (q) 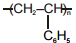 |
| (c) Buna-N | (r) 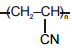 |
| (d) Acrilan | (s) 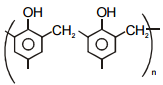 |
| (a) | (b) | (c) | (d) | |
| 1. | (s) | (r) | (q) | (p) |
| 2. | (r) | (p) | (s) | (q) |
| 3. | (p) | (q) | (r) | (s) |
| 4. | (q) | (s) | (p) | (r) |
Monomer of natural rubber is:
1. 1, 3-butadiene
2. Isoprene
3. Styrene
4. Chloroprene
The polymer that can't be prepared by condensation polymerization is :
1. Dacron
2. Nylon-6
3. Glyptal
4. PTFE
In the reaction–
Reagent X is–
1. Triethylaluminium and titanium tetrachloride.
2. Triethyl aluminum.
3. Zeigler Natta Catalyst.
4. Both 1 & 3







