Select the correct option based on statements below:
Assertion (A):
Vulcanisation of rubber is heating of synthetic rubber with sulfur.
Reason (R):
Stiffness is lost in vulcanization.
1.
Both (A) and (R) are true and (R) is the correct explanation of (A).
2.
Both (A) and (R) are true but (R) is not the correct explanation of (A).
3.
(A) is true but (R) is false.
4.
Both (A) and (R) are false.
Match the polymers given in Column-I with their repeating units given in Column-II. Choose the correct option from the codes given below :
| Column-I | Column-II |
| (a) Polystyrene | (p) 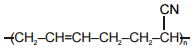 |
| (b) Novolac | (q) 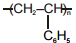 |
| (c) Buna-N | (r) 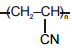 |
| (d) Acrilan | (s) 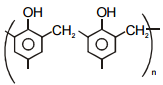 |
| (a) | (b) | (c) | (d) | |
| 1. | (s) | (r) | (q) | (p) |
| 2. | (r) | (p) | (s) | (q) |
| 3. | (p) | (q) | (r) | (s) |
| 4. | (q) | (s) | (p) | (r) |
The polymer that can't be prepared by condensation polymerization is :
1. Dacron
2. Nylon-6
3. Glyptal
4. PTFE
The natural polymer among the following options is:
1. Poly(Butadiene-styrene)
2. Polybutadiene
3. Poly(Butadiene-acrylonitrile)
4. Cis-1,4-polyisoprene
On the basis of mode of formation, polymers can be
classified as:
1. Addition polymers only.
2. Condensation polymers only.
3. Co-polymers.
4. Both addition and condensation polymers.
Which of the following is a chain-growth polymer?
1. proteins
2. starch
3. nucleic acid
4. polystyrene
The repeating units of PTFE are -
1.
2.
3.
4.
Low-density polythene is prepared by -
1. Free-radical polymerization.
2. Cationic polymerization.
3. Anionic polymerization.
4. Ziegler-Natta polymerization.
Which of the following is a synthetic polymer?
1. Phenol-formaldehyde resin.
2. Proteins.
3. Polysaccharides.
4. Natural rubber.
Bakelite is produced by :
1. Phenol, methanol
2. Phenol, NaOH
3. Phenol, urea
4. Phenol, formaldehyde






