Match the polymers given in Column-I with their repeating units given in Column-II. Choose the correct option from the codes given below :
Column-I
Column-II
(a) Polystyrene
(p) 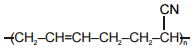
(b) Novolac
(q) 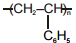
(c) Buna-N
(r) 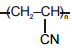
(d) Acrilan
(s) 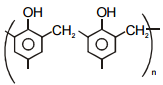
(a)
(b)
(c)
(d)
1.
(s)
(r)
(q)
(p)
2.
(r)
(p)
(s)
(q)
3.
(p)
(q)
(r)
(s)
4.
(q)
(s)
(p)
(r)
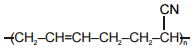
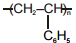
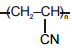

The polymer that can't be prepared by condensation polymerization is :
1. Dacron
2. Nylon-6
3. Glyptal
4. PTFE
The natural polymer among the following options is:
1. Poly(Butadiene-styrene)
2. Polybutadiene
3. Poly(Butadiene-acrylonitrile)
4. Cis-1,4-polyisoprene
On the basis of mode of formation, polymers can be
classified as:
1. Addition polymers only.
2. Condensation polymers only.
3. Co-polymers.
4. Both addition and condensation polymers.
Which of the following is a chain-growth polymer?
1. proteins
2. starch
3. nucleic acid
4. polystyrene
The repeating units of PTFE are -
1.
2.
3.
4.
Low-density polythene is prepared by -
1. Free-radical polymerization.
2. Cationic polymerization.
3. Anionic polymerization.
4. Ziegler-Natta polymerization.
Which of the following is a synthetic polymer?
1. Phenol-formaldehyde resin.
2. Proteins.
3. Polysaccharides.
4. Natural rubber.
Bakelite is produced by :
1. Phenol, methanol
2. Phenol, NaOH
3. Phenol, urea
4. Phenol, formaldehyde
Treatment of rubber with sulfur -
(1) Annealing.
(2) Vulcanization.
(3) Quenching.
(4) None of the above.






