Acrolein is hard, and a high melting point material. The structure of Acrilan among the following is:
1.

2.

3.

4.





Among the following, the monomers which give the polymer neoprene on polymerization are:
| 1. | CH2 = CHCl |
| 2. | CCl2 = CCl2 |
| 3. |  |
| 4. | CF2 = CF2 |
Polymers are examples of
1. High molecular mass macromolecules, which consist of non-repeating structural units derived from monomers
2. How molecular mass macromolecules, which consist of non-repeating structural units derived from monomers
3. High molecular mass macromolecules, which consist of repeating structural units derived from monomers
4. Low molecular mass macromolecules, which consist of repeating structural units derived from monomers
Classification of polymers on the basis of structure include-
1. Linear polymers
2. Branched-chain polymers
3. Cross-linked or Network polymers
4. All of these
The names of monomers of the following polymers are respectively -
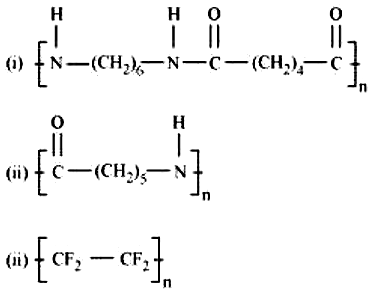
1) Hexaethylenediamine, Caprolactum, Tetrafluoroethene
2) Hexamethylenediamine, Caprolactum, Tetrafluoroethyne
3) Hexamethylenediamine, Caprolactum, Tetrafluoroethene
4) Hexaethylenediamine, Caprolactum, Tetrafluoroethane
Classify the polymers into addition and condensation polymers: Terylene, Bakelite, Polyvinyl chloride, Polythene.
| 1. | Addition polymers: Terylene, bakelite; Condensation polymers: Polyvinyl chloride, polythene |
| 2. | Addition polymers: bakelite; Condensation polymers: Terylene, bakelite, Polyvinylchloride, polythene |
| 3. | Addition polymers: polythene; Condensation polymers: Terylene, bakelite, Polyvinylchloride |
| 4. | Addition polymers: Polyvinyl chloride, polythene; Condensation polymers: Terylene, bakelite |
The difference between Buna-N and Buna-S is :
| 1. | Buna-N is a copolymer of 3−butyne, and acrylonitrile while Buna-S is a copolymer of 3−butyne, and styrene. |
| 2. | Buna-S is a copolymer of 3−butyne, and acrylonitrile while Buna-N is a copolymer of 3−butyne, and styrene. |
| 3. | Buna-N is a copolymer of 1,3−butadiene, and acrylonitrile while Buna-S is a copolymer of 1, 3−butadiene, and styrene. |
| 4. | Buna-S is a copolymer of 1,3−butadiene, and acrylonitrile while Buna-N is a copolymer of 1, 3−butadiene, and styrene. |
On the basis of molecular forces, polymer can be classified as -
1. Elastomers
2. Fibres
3. Thermoplastic polymers
4. All of the above
The term copolymerization means formation of polymer from :
1. The same monomer.
2. Two or more different monomers.
3. Acid and base.
4. Elastomer.
The examples of thermoplastics and thermosetting polymers are respectively :
| 1. | Thermoplastics:polythene, urea-formaldehyde resins ; Thermosetting : bakelite, polystyrene |
| 2. | Thermoplastics: bakelite, polystyrene; Thermosetting : polythene, urea-formaldehyde res |
| 3. | Thermoplastics:polythene, polystyrene ; Thermosetting : bakelite, urea-formaldehyde resins |
| 4. | Thermoplastics:bakelite, urea-formaldehyde resins ; Thermosetting : polythene, polystyrene |






