The examples of thermoplastics and thermosetting polymers are respectively :
1.
Thermoplastics:polythene, urea-formaldehyde resins ; Thermosetting : bakelite, polystyrene
2.
Thermoplastics: bakelite, polystyrene; Thermosetting : polythene, urea-formaldehyde res
3.
Thermoplastics:polythene, polystyrene ; Thermosetting : bakelite, urea-formaldehyde resins
4.
Thermoplastics:bakelite, urea-formaldehyde resins ; Thermosetting : polythene, polystyrene
The monomers used for getting the following polymers are respectively -
(i) Teflon (ii) Bakelite
1. i = Formaldehyde (HCHO) and phenol; ii = Vinyl chloride
2. i = Tetrafluoroethylene; and ii = Vinyl chloride
3. i = Vinyl chloride; ii = Formaldehyde (HCHO), and phenol
4. i = Tetrafluoroethylene; ii = Formaldehyde (HCHO) and phenol
One of the common initiators used in free radical addition polymerization is -
1. Toluene
2. Ethanol
3. Benzoyl peroxide
4. Acetaldehyde
The presence of double bonds in rubber molecules increase -
1. Viscosity
2. Elasticity
3. Surface Tension
4. Vapour pressure
The purpose of vulcanization of rubber is/are -
1. To maintain the elasticity of natural rubber.
2. To improve the tensile strength of natural rubber.
3. To use for a wide range of temperatures.
4. All of the above.
The monomeric repeating units of Nylon-6 and Nylon-6, 6 are respectively -
1. Nylon 6 - Caprolactam; Nylon-6,6 - hexamethylene diamine and adipic acid
2. Nylon 6 - hexamethylene diamine and adipic acid; Nylon-6,6 - Caprolactam
3. Nylon 6 - Caprolactam and adipic acid; Nylon-6,6 - hexamethylene diamine
4. Nylon 6 - pentaethylene diamine and adipic acid; Nylon-6,6 - Caprolactam
The names of the monomer/s of the following polymers are respectively -
(i) Buna-S (ii) Buna-N
(iii) Dacron (iv) Neoprene
1) 1,3-butadiene and styrene, 1,3-butadiene and acrylonitrile, terepthalic acid and ethylene glycol, chloroprene
2) 1,3-butadiene and acrylonitrile, 1,3-butadiene and styrene, chloroprene, terepthalic acid and ethylene glycol
3) 1,3-butadiene and styrene, 1,3-butadiene and acrylonitrile, chloroprene, terepthalic acid and ethylene glycol
4) Chloroprene, terepthalic acid and ethylene glycol, 1,3-butadiene and styrene, 1,3-butadiene and acrylonitrile
The monomer in the following polymeric structures are respectively -
(i)
(ii)
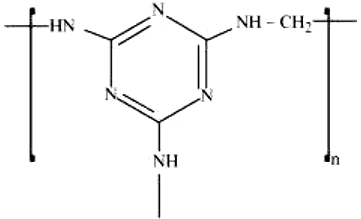
1. Decanoic acid and hexamethylene diamine; melamine and formaldehyde
2. Hexamethylene diamine and formaldehyde; hexamethylene diamine and decanoic acid
3. Decanoic acid and tetramethylene diamine; tetramethylene diamine and formaldehyde
4. Tetramethylene diamine and formaldehyde; decanoic acid and tetramethylene diamine
The salient feature of biodegradable polymer is:
1. Polymer that can be decomposed.
2. Polymer that cannot be decomposed.
3. Polymer that can be denatured.
4. Polymer that cannot be denatured.
The common chain initiator amongst the following in a free radical reaction is :
1. Benzoyl peroxide.
2. Ozone.
3. Chlorine.
4. None of the above.







