Compound (A) C5H10O forms a phenyl hydrazone and gives negative Tollen's and iodoform tests. Compound (A) on reduction gives n-pentane. Compound (A) is:
1. a primary alcohol
2. an aldehyde
3. a ketone
4. a secondary alcohol
RCOOH RCH2OH. This mode of reduction of an acid to alcohol can be affected only by:
1. Zn/HCl
2. Na-alcohol
3. aluminium isopropoxide and isopropyl alcohol
4. LiAlH4
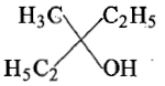
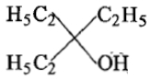
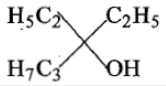
Ethyl ester P. the product P will be:
1. 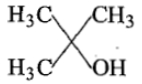

2. 

3. 

4. 

Which of the following has most acidic proton?
1. CH3COCH3
2. (CH3)2C=CH2
3. CH3COCH2COCH3
4. (CH3.CO)3CH
Which one of the following can be oxidised to the corresponding carbonyl compound? [2004]
1. 2-hydroxy propane
2. Ortho-nitro phenol
3. propane
4. 2-methyl-2-hydroxy propane
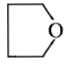
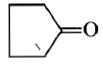
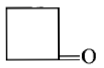
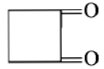
Dry distillation of barium salt of Hexane-1,6-dicarboxylic acid gives:
1. 

2. 

3. 

4. 

Polarisation of electrons in acrolein may be written as
1.
2.
3.
4.
Consider the following reaction;
CH3Br + Mg ABC
compound C is:
1. acetic acid
2. acetaldehyde
3. ethyl alcohol
4. formic acid
The conversion of CH3OH to CH3COOH can be brought in by:
1. K2Cr2O7/H+
2. CO + Rh
3. KMnO4
4. H3PO4
Benzaldehyde and acetaldehyde can be distinguished by:
1. Iodoform test
2. 2,4 - DNP test
3. reaction
4. Wolff-Kishner's reduction






