A metallic bar of Young's modulus \(0.5 \times 10^{11}~\text{Nm}^{-2},\) coefficient of linear thermal expansion \(10^{-5} ~^{\circ}\text{C}^{-1},\) length \(1~\text m\) and cross-sectional area \(10^{-3} ~\text{m}^2\) is heated from \(0^\circ \text{C}\) to \(100^\circ \text C\) without expansion or bending. The compressive force developed in the metallic bar is:
1. \(50 \times 10^3~ \text N\)
2. \(100 \times 10^3 ~\text N\)
3. \(2 \times 10^3~\text N\)
4. \(5 \times 10^3 ~\text N\)
1. \(12^\circ \text{C}\)
2. \(50^\circ \text{C}\)
3. \(73^\circ \text{C}\)
4. \(88.5^\circ \text{C}\)
A cup of coffee cools from \(90^{\circ}\text{C}\) \(80^{\circ}\text{C}\) in \(t\) minutes, when the room temperature is \(20^{\circ}\text{C}.\) The time taken by a similar cup of coffee to cool from \(80^{\circ}\text{C}\) \(60^{\circ}\text{C}\) at room temperature same at \(20^{\circ}\text{C}\) is:
1. \(\frac{10}{13}t\)
2. \(\frac{5}{13}t\)
3. \(\frac{13}{10}t\)
4. \(\frac{13}{5}t\)
The diagram below shows a graphical representation of pressure versus temperature variation for three different low-density gases A, B & C.
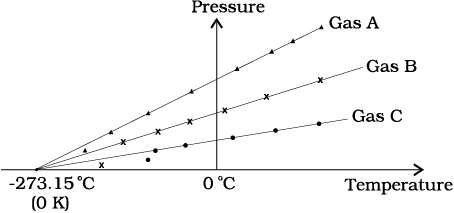
Which of the following can be deduced about absolute zero for the gases A, B & C:
1. Different for all the three gases
2. Same only for gases A & B
3. Same only for gases B & C
4. Same for all the three gases
The figure shows pressure versus temperature graph for a low-density gas kept in a vessel with:
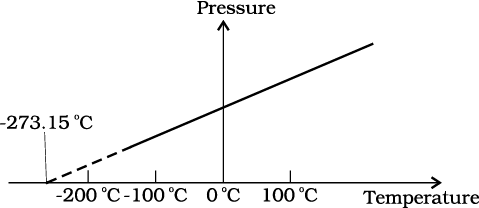
1. Variable volume
2. Volume first increasing then decreasing
3. Volume first decreasing then increasing
4. Constant volume
The given graph shows the variation of Fahrenheit temperature () vs Celsius temperature (). The correct relation between the two temperature scales that can be deduced from the graph below is
An iron bar \(\left(L_{1} = 0 . 1 ~ \text{m}, A_{1} = 0 . 02 ~\text{m}^{2}, K_{1} = 79~\text{W m}^{- 1} \text{K}^{- 1}\right)\) and a brass bar \(\left(L_{2}= 0 . 1 ~\text{m}, A_2 = 0.02~\text{m}^2, K_2 = 109~\text{W m}^{-1}\text{K}^{-1}\right)\) are soldered end to end as shown in the figure. The free ends of the iron bar and brass bar are maintained at \(373\) K and \(273\) K respectively. The heat current through the compound bar is:
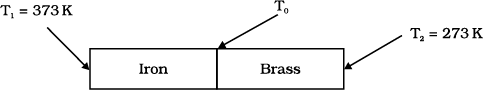
1. \(916.1\) W
2. \(826.1\) W
3. \(926.1\) W
4. \(726\) W
An iron bar \(K_1 = 79~\text{W m}^{-1}\text{K}^{-1}\) and a brass bar \(K_2 = 109~\text{W m}^{-1}\text{K}^{-1}\) are soldered end to end as shown in the figure. The free ends of the iron bar and brass bar are maintained at \(373\) K and \(273\) K respectively. Then the equivalent thermal conductivity of the compound bar is:

| 1. | \(94.6~\text{W m}^{-1}\text{K}^{-1}\) | 2. | \(93.6~\text{W m}^{-1}\text{K}^{-1}\) |
| 3. | \(81.6~\text{W m}^{-1}\text{K}^{-1}\) | 4. | \(91.6~\text{W m}^{-1}\text{K}^{-1}\) |
1. \(50\) s
2. \(52\) s
3. \(42\) s
4. \(48\) s
An iron bar \(\left(L_{1}=0.1 \text{m} , A_{1}=0.02~\text{m}^{2} , K_{1}=79~\text{Wm}^{- 1} \text{K}^{-1}\right)\) and a brass bar \(\left(L_{2}=0.1~\text{m} , A_{2}=0.02~\text{m}^{2} , K_{2}=109~\text{Wm}^{- 1} \text{K}^{- 1}\right)\) are soldered end to end as shown in the figure. The free ends of the iron bar and brass bar are maintained at \(373~ \text{K}\) and \(273~ \text{K}\) respectively. The temperature of the junction of the two bars is:

1. \(215~ \text{K}\)
2. \(315~ \text{K}\)
3. \(415~ \text{K}\)
4. \(115~ \text{K}\)







