(1) sp3 to sp2
(2) sp to sp
(3) sp2 to sp
(4) sp to sp3
The reagent used in dehydrohalogenation process is:
1. Alcoholic KOH
2. NaNH2
3. C2H5ONa
4. All of these
The reaction,
1. CH3CHXCHO
2. CH2XCHCHO
3. CH2=CHCHX2
4. None of these
The reagent used in dehalogenation process is:
1. KOH alc.
2. Zn dust+ alc.
3. Na
4. KOH(aq)
An unknown compound A has a molecular formula C4H6. When A is treated with excess of Br2 a new substance B with formula C4H6Br4 is formed. A forms a white ppt. with ammoniacal silver nitrate solution. A may be-
1. But-1-yne
2. But-2-yne
3. But-1-ene
4. But-2-ene
The appropriate reagent for the following transformation,
 is :
is :
1. Zn(Hg),HCl
2. NH2NH2, OH-
3. H2/Ni
4. NaBH4
Which compound would give 5-keto-2-methyl hexanal upon ozonolysis?
| 1. | 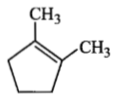 |
2. | 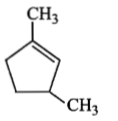 |
| 3. | 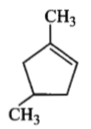 |
4. | 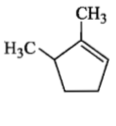 |
The reduction of an alkyne to alkene using Lindlar's catalyst results into:
1. Cis-addition of hydrogen atoms.
2. Trans-addition of hydrogen atoms.
3. A mixture obtained by cis- and trans-additions of hydrogen which are in equilibrium with each other.
4. A mixture obtained by cis- and trans-additions of hydrogen atoms which are not in equilibrium with each other.
Identify Z in the sequence:
\(\mathrm{CH}_ 3-\mathrm{CH}_2-\mathrm{CH}=\mathrm{CH}_2 \xrightarrow{\mathrm{HBr} / \mathrm{H}_2 \mathrm{O}_2} \mathrm{Y} \xrightarrow{\mathrm{C}_2 \mathrm{H}_5 \mathrm{O}^{-}\mathrm{Na}^{+}} \mathrm{Z} \)
| 1. |  |
| 2. |  |
| 3. | \( \mathrm{CH}_3-\left(\mathrm{CH}_2\right)_3-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3 \) |
| 4. | \( \mathrm{CH}_3-\left(\mathrm{CH}_2\right)_4-\mathrm{O}-\mathrm{CH}_3 \) |
Acetylene can be converted to higher alkyne using the following sequence of reactions:
(1) Na,RX
(2) RMg X,R X
(3) either of these two
(4) none of these






