Synthetic detergents are better than soaps because:
1.
Synthetic detergents work both in soft water and hard water.
2.
Soaps work both in soft water and hard water.
3.
Synthetic detergents work only in hard water.
4.
Soaps work only in hard water.
Choose the correct statement:
| 1. | Detergents that can not be degraded by bacteria are called biodegradable detergents. |
| 2. | Biodegradable detergents have straight hydrocarbon chains. |
| 3. | Detergents that can be degraded by bacteria are called non-biodegradable detergents. |
| 4. | Biodegradable detergents have highly branched hydrocarbon chains. |
Soaps do not work in hard water because:
| 1. | Calcium and magnesium ions displace sodium or potassium from their salts and form insoluble calcium or magnesium salts of fatty acids. |
| 2. | Sodium or potassium ions displace calcium and magnesium from their salts and form insoluble calcium or magnesium salts of fatty acids. |
| 3. | Calcium and magnesium ions displace sodium or potassium from their salts and form insoluble sodium or potassium salts of fatty acids. |
| 4. | None of the above. |
Out of soaps and synthetic detergents, ______ are used to check the hardness of water as -
1. Soaps, get precipitated in hard water, but not in soft water
2. Soaps, get precipitated in soft water, but not in hard water
3. Synthetic detergents, get precipitated in hard water, but not in soft water
4. Synthetic detergents, get precipitated in soft water, but not in hard water
Detergents are preferred over soap for cleaning clothes because -
1. Detergents form soluble salts.
2. Detergents form insoluble salts.
3. Soaps form soluble salts.
4. Soaps form insoluble salts of potassium.
Drugs can be classified on the basis of:
1. Pharmacological effect.
2. Chemical structure.
3. Molecular targets.
4. All of the above.
Cetyltrimethylammonium bromide is an example of:
1. Cationic detergents
2. Anionic detergents
3. Non-ionic detergents
4. None of the above
The correct statement among the following regarding cationic detergent is/are:
| 1. | These are sodium salts of long chain alcohols or alkylbenzenesulphonic acids. |
| 2. | Sodium 4-(1-dodecyl) benzenesulphonate (SDS) is an example of an anionic detergent. |
| 3. | These are quaternary ammonium salts of acetates, chlorides, or bromides of amine. |
| 4. | None of the above. |
The correct statement among the following during the cleansing action of soaps is:
| 1. | Hydrophobic parts of the stearate ions attach themselves to the oil droplets. |
| 2. | Hydrophilic parts of the stearate ions project outside the oil droplets. |
| 3. | Hydrophilic parts of the stearate ions attach themselves to the oil droplets. |
| 4. | Both (1) and (2) |
The correctly labelled compound(s) is/are-
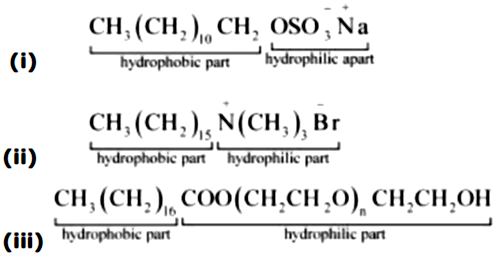
(iv). All of these






