100 g of ice (latent heat 80 cal g-1) at 0 °C is mixed with 100 g of water (specific heat 1 cal g-1 °C-1) at 80 °C. The final temperature of the mixture will be:
1. 0 °C
2. 40 °C
3. 80 °C
4. <0 °C
200 g of ice at -20°C is mixed with 500 g of water at 20°C in an insulating vessel. Final mass of water in vessel is: (Specific heat of ice)
1. 700 g
2. 600 g
3. 400 g
4. 200 g
Which of the following material is most suitable for cooking utensils?
1. Low conductivity and low specific heat
2. High conductivity and low specific heat
3. Low conductivity and high specific heat
4. High conductivity and high specific heat
Which of the following material is used to make a calorimeter?
1. Glass
2. Ebonite
3. Metal
4. Superconductor
The thermal capacity of 100 g of aluminium: (specific heat)
1. 0.002 cal/°C
2. 20 cal/°C
3. 200 cal/°C
4. 100 cal/°C
The density of water is maximum at:
1. 39.2 °F
2. 4 °F
3. 0 °C
4. 273 K
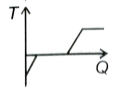
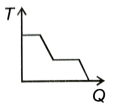
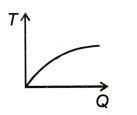
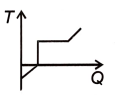
A block of ice at -12 °C is slowly heated and converted into steam at 100 °C. Which of the following curves best represents the event?
1.
2.
3.
4.
In engines, water is used as a coolant, because:
1. It is a good conductor of heat energy.
2. It has a low density.
3. It has high specific heat.
4. It is a bad conductor of heat energy.
Select correct statement related to heat.
1. Heat is possessed by a body.
2. Hot water contains more heat as compared to cold water.
3. Heat is energy which flows due to temperature difference.
4. All of these
Which of the following factors affect the thermal conductivity of a rod?
1. Area of cross-section
2. Length of rod
3. Material of rod
4. All of these






