From a certain apparatus, the diffusion rate of hydrogen has an average value of 28.7 . The diffusion of another gas under the same conditions is measured to have an average rate of 7.2 . The unknown gas is:
1. Oxygen
2. Nitrogen
3. Helium
4. None of these
1. Oxygen
2. Nitrogen
3. Helium
4. None of these
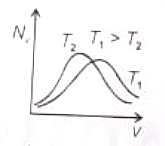
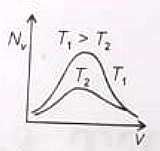
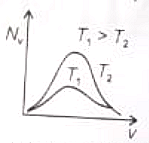
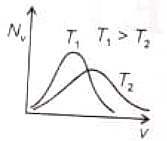
The effect of temperature on Maxwell's speed distribution is correctly shown by:
(1) 
(2) 
(3) 
(4) 
Select the incorrect statement about Maxwell's speed distribution curve.
(1) The distribution function depends only on the absolute temperature.
(2)
(3) The area under the distribution curve gives the total number of molecules of the gas.
(4) The distribution curve is symmetric about the most probable speed.
By increasing the temperature of a gas by 6 oC, its pressure increases by 0.4% at constant volume. Then the initial temperature of the gas is:
(1) 1000 K
(2) 2000 K
(3) 1500 K
(4) 750 K
Boyle's law is obeyed by:
(1) Real gas of constant mass and temperature.
(2) Ideal gas of constant mass and temperature.
(3) Both ideal and real gases at constant temperature and variable mass.
(4) Both ideal and real gases of constant mass and variable temperature.
For an ideal gas, the fractional change in its volume per degree rise in temperature at constant pressure is equal to [T is the absolute temperature of the gas]:
(1) T0
(2) T
(3) T-1
(4) T2
The rise in the temperature of a given mass of an ideal gas at constant pressure and at temperature 27 oC to double its volume is:
(1) 327oC
(2) 54oC
(3) 300oC
(4) 600oC
A container has N molecules at absolute temperature T. If the number of molecules is doubled but kinetic energy in the box remains the same as before, the absolute temperature of the gas is:
(1) T
(2)
(3) 3T
(4) 4T
During an experiment, an ideal gas is found to obey an additional law VP2 = constant. The gas is initially at temperature T and volume V. When it expands to volume 2V, the resulting temperature is:
(1)
(2) 2T
(3)
(4)
When the pressure remaining constant, at what temperature, will the r.m.s. speed of gas molecules increases by 10% of the r.m.s. the speed at NTP?
(1) 81.5 K
(2) 81.5 oC
(3) 815.3 K
(4) -81.5 oC






