An oxygen cylinder of volume 30 litres has an initial gauge pressure of 15 atm and a temperature of 27 °C. After some oxygen is withdrawn from the cylinder, the gauge pressure drops to 11 atm, and its temperature drops to 17 °C. The mass of oxygen taken out of the cylinder is:
1. 0.14 kg
2. 0.16 kg
3. 0.18 kg
4. 0.21 kg
2. 0.16 kg
3. 0.18 kg
4. 0.21 kg
An air bubble of volume 1.0 rises from the bottom of a lake 40 m deep at a temperature of 12 °C. To what volume does it grow when it reaches the surface, which is at a temperature of 35 °C?
1. 5.3 cm3
2. 4.0 cm3
3. 3.7 cm3
4. 4.9 cm3
What is the total number of air molecules (inclusive of oxygen, nitrogen, water vapor, and other constituents) in a room of capacity \(25.0\) m3 at a temperature of \(27^\circ \text C\) and \(1\) atm pressure?
1. \(6.1\times10^{23}\) molecules
2. \(6.1\times10^{26}\) molecules
3. \(7.1\times10^{23}\) molecules
4. \(7.1\times10^{26}\) molecules
| 1. | \(11 . 21 \times 10^{- 20}~\text{J}\) | 2. | \(3 . 09 \times 10^{- 16}~\text{J}\) |
| 3. | \( 6 . 21 \times 10^{- 21} ~\text{J} \) | 4. | \(5 . 97 \times 10^{- 19}~\text{J}\) |
At what temperature is the root mean square speed of an atom in an argon gas cylinder equal to the RMS speed of a helium gas atom at \(-20^\circ \text{C}?\)
(Given the atomic mass of \(\mathrm{Ar}=39.9~\text{u}\) and of \(\mathrm{He}=4.0~\text{u}\))
1. \(1.01 \times 10^3 ~\text{K} \)
2. \(3.15 \times 10^3 ~\text{K} \)
3. \(1.91 \times 10^3~ \text{K} \)
4. \(2.52 \times 10^3 ~\text{K}\)
From a certain apparatus, the diffusion rate of hydrogen has an average value of \(28.7~\text{cm}^3 \text{s}^{-1}.\) The diffusion of another gas under the same conditions is measured to have an average rate of \(7.2~\text{cm}^3 \text{s}^{-1}.\) The unknown gas is:
1. Oxygen
2. Nitrogen
3. Helium
4. None of these
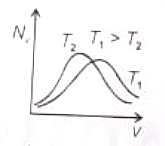
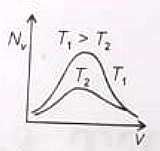
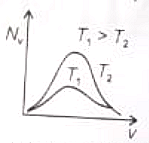
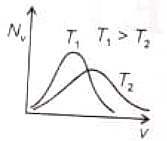
The effect of temperature on Maxwell's speed distribution is correctly shown by:
(1) 
(2) 
(3) 
(4) 
Select the incorrect statement about Maxwell's speed distribution curve.
(1) The distribution function depends only on the absolute temperature.
(2)
(3) The area under the distribution curve gives the total number of molecules of the gas.
(4) The distribution curve is symmetric about the most probable speed.
By increasing the temperature of a gas by 6 oC, its pressure increases by 0.4% at constant volume. Then the initial temperature of the gas is:
(1) 1000 K
(2) 2000 K
(3) 1500 K
(4) 750 K
Boyle's law is obeyed by:
(1) Real gas of constant mass and temperature.
(2) Ideal gas of constant mass and temperature.
(3) Both ideal and real gases at constant temperature and variable mass.
(4) Both ideal and real gases of constant mass and variable temperature.






