The polymer containing strong intermolecular forces like hydrogen bonding is
(1) Teflon
(2) nylon 6,6
(3) polystyrene
(4) natural rubber
The species which can best serve as an initiator for the cationic polymerization is
(1)
(2)
(3)
(4) BuLi
Which polymer is used in the manufacture of paints and lacquers?
(1) Bakelite
(2) Glyptal
(3) Polypropene
(4) Polyvinyl chloride
Which of the following statements about low-density polythene is false?
1. It is a poor conductor of eléctricity
2. Its synthesis requires oxygen or Peroxide indicator as a Catalyst
3. Its synthesis requires high Pressure
4. It is used in the manufacture of buckets, etc.
Passage
The polymer is a very large molecule that is made up of repeating small molecular units called monomers. The chemical reaction that unites the monomers is called polymerization. There are homopolymers, copolymers depending upon the type of monomers present in them.
Which of the following monomers will give radical polymerization most readily?
1.
2.
3.
4.
Which of the following cannot be used as a plasticizer?
1. 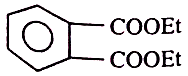
2. 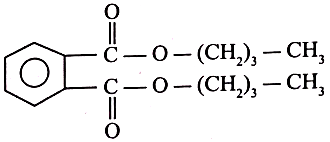
3. cresyl triphosphate
4. Di- n-octyl phthalate
Which of the following are biopolymers?
(a) Leather
(b) Nucleic acids
(c) Orlon
(d) Bakelite
1. a,b
2.a,b,c
3.b
4.a
Which of the following compounds not contain amide linkage/group?
(1) Acetamide
(2) Nylon-6
(3) Cellulose
(4) Proteins
Incorrect statement regarding the polymers is:
(1) They all have a repeating structural unit.
(2) They have high viscosity.
(3) They scatter light.
(4) They have low molecular weights.
Polymers that involve cross-linkages are:
(I) Bakelite
(II) High density Polythene
(III) PVC
(IV) Vulcanised rubber
(1) I, II, IV
(2) II only
(3) III, IV
(4) I, IV






