A nucleus with Z = 92 emits the following in a sequence:
. The Z of the resulting nucleus is [AIEEE 2003]
1. 74
2. 76
3. 78
4. 82
In nuclear fission, the percentage of mass converted into energy is about
1. 1%
2. 0.1%
3. 0.01%
4. 10%
The mass number of helium is 4 and that for suphur is 32. The radius of sulphur nuclei is larger than that of helium by
1.
2. 4
3. 2
4. 8
:
1. 11, 12, 0
2. 23, 12, 11
3. 12, 11, 0
4. 23, 11, 12
A thorium nucleus is formed when a uranium nucleus emits an . The atomic number of thorium is
1. 92
2. 90
3. 82
4. 94
The activity of the radioactive element decreases to one-third of the original activity in a period of 7 years. After a further lapse of 7 years, its activity will be:
1.
2.
3.
4.
The radius of Germanium (Ge) nuclide is measured to be twice the radius of . The number of nucleons in Ge is:
1. 73
2. 74
3. 75
4. 72
if the binding energies of \({}_{1}^{2}\mathrm{H}, {}_{1}^{3}\mathrm{H},\) and \({}_{2}^{4}\mathrm{He}\) are respectively \(a,b,\) and \(c\) (in MeV), then the energy in (MeV) released in this reaction is:
1. \(c+a-b\)
2. \(c-a-b\)
3. \(a+b+c\)
4. \(a+b-c\)
A radioactive nucleus X decays to a stable nucleus 'y'. The graph of the rate of formation of 'y' against time 't' will be:
| 1. | 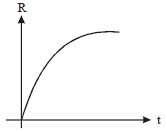  |
2. | 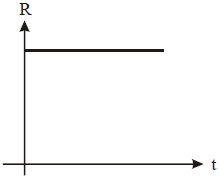  |
| 3. | 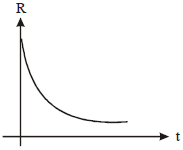  |
4. | 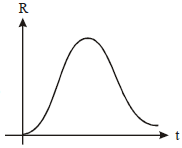  |






