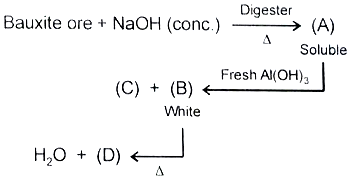
Compound (D) is
1.
2.
3.
4.

Compound (D) is
Consider the following metallurgical process
The various processes A, B, and C respectively
1. Calcination, smelting, and electrolysis
2. Roasting, smelting, and electrolysis
3. Calcination, auto reduction, and bassemetisation
4. Roasting, aluminothermic reduction, electrolysis
NaCN is a depressant that is used:
| 1. | To Separate PbS from ZnS by forming a complex with PbS. |
| 2. | To Separate PbS from ZnS by forming a complex with ZnS. |
| 3. | To Form froth. |
| 4. | As a collector. |
Ellingham's diagram for the formation of is a straight line in the given graph.
This is due to:
1. Increase in entropy during formation.
2. Decrease in entropy during formation.
3. Entropy remains constant during formation.
4. Can not be predicted.
Which of the following statements is correct regarding the slag obtained during the
extraction of a metal like copper or iron?
(1) The slag is lighter and has a lower melting point than the metal
(2) The slag is heavier and has a lower melting point than the metal
(3) The slag is lighter and has a higher melting point than the metal
(4) The slag is heavier and has a higher melting point than the metal
The process of bringing the metal or its ore into solution by the action of a suitable
chemical reagent followed by extraction of the metal either by electrolysis or by a
the suitable precipitating agent is called
1. Electrometallurgy
2. Hydrometallurgy
3. Electrorefining
4. Zone refining
Why is roasting important in the iron metallurgy process?
| 1. | FeO gets oxidized to Fe2O3 which does not combine with acidic SiO2 |
| 2. | SiO2 is an acidic oxide which can combine with metallic oxides i.e. FeO, and Fe2O3 |
| 3. | FeO when roasted get oxidized to more basic Fe2O3 |
| 4. | None of the above. |
Identify the metal M, which has the following extraction principle.
1. Mg
2. Pb
3. Sn
4. Fe
The process of converting hydrated alumina into anhydrous alumina is called
1. Roasting
2. Smelting
3. Dressing
4. Calcination
Mg in the Kroll process acts as
(1) Reducing agent
(2) Oxidizing agent
(3) Oxidizing agent at low temperature
(4) Reducing agent at low temperature







