A Carnot engine has an efficiency of \(50\%\). If the temperature of sink is reduced by \(40^{\circ}\text{C}\), its efficiency increases by \(30\%\). The temperature of the source will be:
1. \(166.7\) K
2. \(255.1\) K
3. \(266.7\) K
4. \(367.7\) K
1. \(166.7\) K
2. \(255.1\) K
3. \(266.7\) K
4. \(367.7\) K
Subtopic: Carnot Engine |
57%
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
In the first case, a Carnot engine operates between temperatures of \(300~\text{K}\) and \(100~\text{K}.\) In the second case, as shown in the figure, two heat engines are connected in series (i.e., the output of the first engine becomes the input for the second engine).
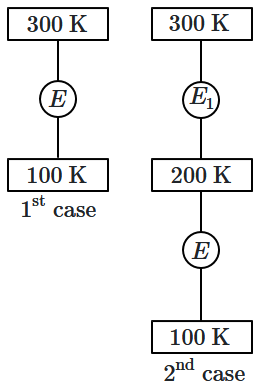
What is the overall efficiency of the combined engine system in the second case?
| 1. | Same as the first case. |
| 2. | Always greater than the first case. |
| 3. | Always less than the first case. |
| 4. | May increase or decrease with respect to the first case. |
Subtopic: Carnot Engine |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
An ideal gas forms the working substance of a Carnot engine, and is taken around the Carnot cycle. We form the integral: \(I=\int\dfrac{dQ}{T},\)
where \(dQ\) is the heat supplied to the gas and \(T\) is the temperature of the gas. The integral is evaluated over the entire cycle. The value of the integral \(I\) is:
where \(dQ\) is the heat supplied to the gas and \(T\) is the temperature of the gas. The integral is evaluated over the entire cycle. The value of the integral \(I\) is:
| 1. | zero |
| 2. | negative |
| 3. | positive |
| 4. | non-negative(positive or zero) |
Subtopic: Carnot Engine |
50%
Level 3: 35%-60%
Hints
Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R):
In the light of the above statements choose the correct answer from the options given below:
| Assertion (A): | At a sink temperature of \(-273^{\circ}\) C, the efficiency of a Carnot engine will be 1. |
| Reason (R): | The efficiency of a Carnot engine is given by \(\eta=1-{{T_{\sin k}}\over{T_{source}}}\) |
In the light of the above statements choose the correct answer from the options given below:
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |
Subtopic: Carnot Engine |
73%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
A Carnot engine operates between temperatures of \(27^\circ \mathrm C\) and \(127^\circ \mathrm C\) and performs \(2\) kJ of work. The amount of heat energy rejected is:
| 1. | \(4\) kJ | 2. | \(6\) kJ |
| 3. | \(8\) kJ | 4. | \(12\) kJ |
Subtopic: Carnot Engine |
55%
Level 3: 35%-60%
JEE
Please attempt this question first.
Hints
Please attempt this question first.
A Carnot engine takes \(5000~\text{kcal}\) of heat from a reservoir at \(727^\circ \text{C}\) and gives heat to a sink at \(127^\circ \text{C}.\) The work done by the engine is:
1. \(3 \times 10^6 ~\text J\)
2. zero
3. \(12.6 \times 10^6 \)
4. \(8.4 \times 10^6 \)
1. \(3 \times 10^6 ~\text J\)
2. zero
3. \(12.6 \times 10^6 \)
4. \(8.4 \times 10^6 \)
Subtopic: Carnot Engine |
61%
Level 2: 60%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.




