Select Chapter Topics:
For the \(P\text-V\) diagram given for an ideal gas, out of the following which one correctly represents the \(T\text-P\) diagram?

1.

3.

2.

4.





Subtopic: Types of Processes |
58%
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
A carnot's engine works as a refrigerator between \(250~\text{K}\) and \(300~\text{K}.\) It receives \(500~ \text{cal}\) heat from the reservoir at a lower temperature. The amount of work done in each cycle to operate the refrigerator is:
1. \(772 ~\text{J}\)
2. \(420 ~\text{J}\)
3. \(2100 ~\text{J}\)
4. \(2520 ~\text{J}\)
1. \(772 ~\text{J}\)
2. \(420 ~\text{J}\)
3. \(2100 ~\text{J}\)
4. \(2520 ~\text{J}\)
Subtopic: Carnot Engine |
72%
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
One mole of an ideal monatomic gas is compressed isothermally in a rigid vessel to double its pressure at room temperature \(27^\circ \text C.\) The work done on the gas will be :
1. \(300R\)
2. \(300R ~\mathrm{ln}(2)\)
3. \(300 ~\mathrm{ln}(6)\)
4. \(300R~\mathrm{ln}(7)\)
1. \(300R\)
2. \(300R ~\mathrm{ln}(2)\)
3. \(300 ~\mathrm{ln}(6)\)
4. \(300R~\mathrm{ln}(7)\)
Subtopic: Work Done by a Gas |
80%
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
An engine operates by taking n moles of an ideal gas through the cycle \(ABCDA\) shown in the figure. The thermal efficiency of the engine is:
(Take \({C}_v=1.5{R},\) where \({R}\) is gas constant)

1. \(0.32\)
2. \(0.15\)
3. \(0.24\)
4. \(0.08\)
(Take \({C}_v=1.5{R},\) where \({R}\) is gas constant)

1. \(0.32\)
2. \(0.15\)
3. \(0.24\)
4. \(0.08\)
Subtopic: Carnot Engine |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
An ideal gas goes through a reversible cycle \({a}\rightarrow{b}\rightarrow{c}\rightarrow{d}\) has the \({V \text- T}\) diagram as shown below. Process \({d}\rightarrow{a}\) and \({b}\rightarrow{c}\) are adiabatic.

The corresponding \({P \text- V}\) diagram for the process is (all figures are schematic and not drawn to scale):

The corresponding \({P \text- V}\) diagram for the process is (all figures are schematic and not drawn to scale):
| 1. |  |
3. |  |
| 2. |  |
4. |  |
Subtopic: Cyclic Process |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
An experiment takes \(10\) minutes to raise the temperature of water in a container from \({0}^\circ \text{C}\) to \({100}^\circ\text{C}\) and another \(55\) minutes to convert it totally into steam by a heater supplying heat at a uniform rate. Neglecting the specific heat of the container and taking the specific heat of the water to be \({1}~\text{cal/g}^\circ \text{C},\) the heat of vapourization according to this experiment will come out to be:
1. \({560}~\text{cal/g}\)
2. \({550}~\text{cal/g}\)
3. \({540}~\text{cal/g}\)
4. \({530}~\text{cal/g}\)
1. \({560}~\text{cal/g}\)
2. \({550}~\text{cal/g}\)
3. \({540}~\text{cal/g}\)
4. \({530}~\text{cal/g}\)
Subtopic: Molar Specific Heat |
50%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
A gas undergoes a cyclic-process \(ABCA\) as shown in the figure. Then the work done by the gas for \(A\rightarrow B\rightarrow C\) is:
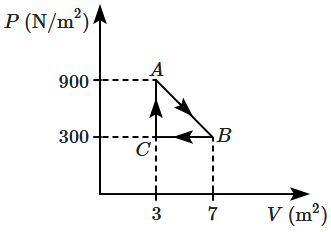
1. \(1800~\text{J}\)
2. \(1200~\text{J}\)
3. \(3600~\text{J}\)
4. \(600~\text{J}\)

1. \(1800~\text{J}\)
2. \(1200~\text{J}\)
3. \(3600~\text{J}\)
4. \(600~\text{J}\)
Subtopic: Work Done by a Gas |
93%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
The specific heat at constant pressure of a real gas obeying \(\mathrm{PV}^2=\mathrm{RT}\) equation is:
1. \(\frac{R}{3}+C_v\)
2. \(R\)
3. \(C_v+R\)
4. \(C_v+R / 2 V\)
1. \(\frac{R}{3}+C_v\)
2. \(R\)
3. \(C_v+R\)
4. \(C_v+R / 2 V\)
Subtopic: Molar Specific Heat |
55%
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
A total of \(48\) J heat is given to one mole of helium kept in a cylinder. The temperature of helium increases by \(2 °C\). The work done by the gas is :
(Given : \(\mathrm{R}=8.31 \mathrm{Jk}^{-1} \mathrm{~mol}^{-1}\))
(Given : \(\mathrm{R}=8.31 \mathrm{Jk}^{-1} \mathrm{~mol}^{-1}\))
1. \(48\) J
2. \(23.1\) J
3. \(24.9\) J
4. \(72.9\) J
Subtopic: Work Done by a Gas |
87%
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
The volume of an ideal gas \((y-1.5)\) is changed adiabatically from \(5\) litres to \(4\) litres. The ratio of initial pressure to final pressure is :
1. \(\frac{4}{5}\)
2. \(\frac{8}{5 \sqrt{5}}\)
3. \(\frac{16}{25}\)
4. \(\frac{2}{\sqrt{5}}\)
1. \(\frac{4}{5}\)
2. \(\frac{8}{5 \sqrt{5}}\)
3. \(\frac{16}{25}\)
4. \(\frac{2}{\sqrt{5}}\)
Subtopic: Types of Processes |
81%
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.




