
1. Dichloromethyl cation \((^{+}CHCl_{2})\)
2. Formyl cation \((^{+}CHO)\)
3. Dichloromethyl anion \((^{-}CHCl_{2})\)
4. Dichlorocarbene (:CCl2)
Carboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to:
| 1. | Formation of intramolecular H-bonding |
| 2. | Formation of carboxylate ion |
| 3. | More extensive association of carboxylic acid via van der waals force of attraction |
| 4. | Formation of intermolecular H-bonding |
A, X, Y, and Z in the above-mentioned reaction are:
| A | X | Y | Z | |
| 1. | Methoxy- methane |
Ethanol | Ethanoic acid | Semicarbazone |
| 2. | Ethanal | Ethanol | But - 2 - enal | semicarbazone |
| 3. | Ethanol | Acetaldehyde | Butanone | Hydrazone |
| 4. | Methoxy- methane |
Ethanoic acid | Acetate | Hydrazine |
Which product is generated when cyclohexanone undergoes aldol condensation and is subsequently heated?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
In the below reaction, the structure of "A" is:
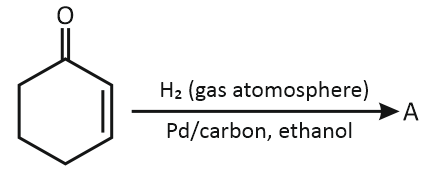
| 1. |  |
2. |  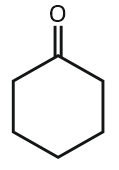 |
| 3. |  |
4. |  |
What is the correct order of the carboxylic acids' strength?
| I. |  |
II. |  |
III. |  |
| 1. | I > II > III | 2. | II > III > I |
| 3. | III > II > I | 4. | II > I > III |
The correct statement regarding a carbonyl compound with a hydrogen atom on its alpha-carbon is:
| 1. | A carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as aldehyde-ketone equilibration. |
| 2. | A carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as carbonylation. |
| 3. | A carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as keto-enol tautomerism. |
| 4. | A carbonyl compound with a hydrogen atom on its alpha-carbon never equilibrates with its corresponding enol. |
The product formed by the reaction of an aldehyde with a primary amine is:
1. Ketone
2. Carboxylic acid
3. Aromatic acid
4. Schiff base








