Select Chapter Topics:
The correct statement regarding the comparison of staggered and eclipsed conformations of ethane is:
| 1. | The eclipsed conformation of ethane is more stable than staggered conformation because eclipsed conformation has no torsional strain. |
| 2. | The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has a torsional strain. |
| 3. | The staggered conformation of ethane is more stable than eclipsed conformation because staggered conformation has no torsional strain. |
| 4. | The staggered conformation of ethane is less stable than eclipsed conformation because staggered conformation has a torsional strain. |
Subtopic: Aliphatic Hydrocarbon -Nomenclature, Isomerism & Mechanism |
84%
Level 1: 80%+
NEET - 2016
Hints
Links
The most suitable reagent for the following conversion is-
| 1. | Hg2+/ H+, H2O | 2. | Na/liquid NH3 |
| 3. | H2, Pd/C, quinoline | 4. | Zn/HCl |
Subtopic: Aliphatic Hydrocarbon -Nomenclature, Isomerism & Mechanism |
81%
Level 1: 80%+
NEET - 2019
Hints
Links
\(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}=\mathrm{CH}_2 \xrightarrow[\text { peroxide}]{\mathrm{HBr}} Y \xrightarrow{\mathrm{C}_2 \mathrm{H}_5 \mathrm{ONa}} Z\)
Product \(Z\) in the above-mentioned reaction is:
1. \(\mathrm{CH}_3-\left(\mathrm{CH}_2\right)_3-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3\)
2. \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3\)
3. \(\mathrm{CH}_3\left(\mathrm{CH}_2\right)_4-\mathrm{O}-\mathrm{CH}_3\)
4. \(\mathrm{CH}_3 \mathrm{CH}_2-\mathrm{CH}\left(\mathrm{CH}_3\right)-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3 \)
Product \(Z\) in the above-mentioned reaction is:
1. \(\mathrm{CH}_3-\left(\mathrm{CH}_2\right)_3-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3\)
2. \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3\)
3. \(\mathrm{CH}_3\left(\mathrm{CH}_2\right)_4-\mathrm{O}-\mathrm{CH}_3\)
4. \(\mathrm{CH}_3 \mathrm{CH}_2-\mathrm{CH}\left(\mathrm{CH}_3\right)-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3 \)
Subtopic: Aliphatic Hydrocarbon -Nomenclature, Isomerism & Mechanism |
74%
Level 2: 60%+
AIPMT - 2014
Hints
Links
The incorrect IUPAC name among the following is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Subtopic: Aliphatic Hydrocarbon -Nomenclature, Isomerism & Mechanism |
72%
Level 2: 60%+
AIPMT - 2012
Hints
Links
Which compound among the following has the highest boiling point?
| 1. | Iso-octane | 2. | n-Octane |
| 3. | 2,2,3,3-Tetramethyl butane | 4. | n-Butane |
Subtopic: Aliphatic Hydrocarbon- Physical Properties |
82%
Level 1: 80%+
Hints
Which of the following hydrocarbon fuels has the highest octane rating?
1. Methane
2. Ethane
3. Iso-octane
4. Triptane
Subtopic: Aliphatic Hydrocarbon- Physical Properties |
76%
Level 2: 60%+
Hints
undergoes Wurtz reaction to give-
| 1. | Propane + Ethane | 2. | Propane |
| 3. | Propane + Ethane + Butane | 4. | Propane + Butane |
Subtopic: Aliphatic Hydrocarbon - Methods of Preparation |
81%
Level 1: 80%+
Hints
The main product A and B in the above mentioned reaction are respectively-
1.
2.
3. 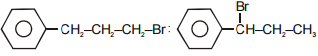
4. 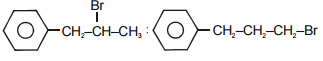
Subtopic: Aliphatic Hydrocarbon - Methods of Preparation |
82%
Level 1: 80%+
Hints
3-Hexyne can be converted to trans-3-Hexene by the action of:
1. - Pd/
2. Li-Liq.
3. - Pt
4.
Subtopic: Aliphatic Hydrocarbon - Methods of Preparation |
80%
Level 1: 80%+
Hints
The major product in the above-mentioned reaction is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Subtopic: Aliphatic Hydrocarbon - Methods of Preparation |
75%
Level 2: 60%+
Hints
Select Chapter Topics:











