Select Chapter Topics:
Using the rules for significant figures, the correct answer for the expression \(\frac{0.02858 \times 0.112}{0.5702}\) will be :
1. \(0.005613\)
2. \(0.00561\)
3. \(0.0056\)
4. \(0.006\)
Subtopic: Introduction |
80%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
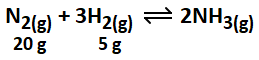
Consider the above reaction, the limiting reagent of the reaction and number of moles of NH3 formed respectively are:
1. \(\mathrm{H}_2, 1.42 \text { moles }\)
2. \(\mathrm{H}_2, 0.71 \text { moles }\)
3. \(\mathrm{N}_2, 1.42 \text { moles }\)
4. \(\mathrm{N}_2, 0.71 \text { moles }\)
Subtopic: Moles, Atoms & Electrons |
79%
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
Compound A contains \(8.7\%\) Hydrogen, \(74\%\) Carbon and \(17.3\%\) Nitrogen. The molecular formula of the compound is:
(Given : Atomic masses of \(\mathrm{C, H}\) and \(\mathrm{N}\) are \(12, 1\) and \(14\) amu respectively. The molar mass of the compound A is \(\mathrm{162 ~g ~mol^{–1}}\))
1. \(\mathrm{C_4H_6N_2}\)
2. \(\mathrm{C_2H_3N}\)
3. \(\mathrm{C_5H_7N}\)
4. \(\mathrm{C_{10}H_{14}N_2}\)
(Given : Atomic masses of \(\mathrm{C, H}\) and \(\mathrm{N}\) are \(12, 1\) and \(14\) amu respectively. The molar mass of the compound A is \(\mathrm{162 ~g ~mol^{–1}}\))
1. \(\mathrm{C_4H_6N_2}\)
2. \(\mathrm{C_2H_3N}\)
3. \(\mathrm{C_5H_7N}\)
4. \(\mathrm{C_{10}H_{14}N_2}\)
Subtopic: Empirical & Molecular Formula |
78%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Consider the reaction
\(\small\begin{aligned} 4 \mathrm{HNO}_3(\mathrm{l}) +3 \mathrm{KCl}(\mathrm{s}) \ \rightarrow \mathrm{Cl}_2(\mathrm{~g})+\mathrm{NOCl}(\mathrm{g})+ \\2 \mathrm{H}_2 \mathrm{O}(\mathrm{g})+3 \mathrm{KNO}_3(\mathrm{~s}) \end{aligned}\)
The amount of HNO3 required to produce 110.0 g of KNO3 is:
(Given: Atomic masses of H, O, N and K are 1, 16, 14 and 39 respectively).
1. 32.2 g
2. 69.4 g
3. 91.5 g
4.162.5 g
\(\small\begin{aligned} 4 \mathrm{HNO}_3(\mathrm{l}) +3 \mathrm{KCl}(\mathrm{s}) \ \rightarrow \mathrm{Cl}_2(\mathrm{~g})+\mathrm{NOCl}(\mathrm{g})+ \\2 \mathrm{H}_2 \mathrm{O}(\mathrm{g})+3 \mathrm{KNO}_3(\mathrm{~s}) \end{aligned}\)
The amount of HNO3 required to produce 110.0 g of KNO3 is:
(Given: Atomic masses of H, O, N and K are 1, 16, 14 and 39 respectively).
1. 32.2 g
2. 69.4 g
3. 91.5 g
4.162.5 g
Subtopic: Moles, Atoms & Electrons |
82%
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
On complete combustion of 0.492 g of an organic compound containing C, H and O, 0.7938 g of \(\mathrm{CO}_2\) and 0.4428 g of \(\mathrm{H}_ 2 \mathrm{O}\) was produced. The % composition of oxygen in the compound is:
| 1. | 46% | 2. | 53% |
| 3. | 32% | 4. | 76% |
Subtopic: Empirical & Molecular Formula |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
A commercially sold conc. \(\text{HCl}\) is \(35\%\) \(\text{HCl}\) by mass. If the density of this commercial acid is \(1.46~\text{g/mL},\) the molarity of this solution is :
(Atomic mass: \(\text{Cl = 35.5 amu, H = 1 amu)}\)
1. \(10.2~\text{M}\)
2. \(12.5~\text{M}\)
3. \(14.0~\text{M}\)
4. \(18.2 ~\text{M}\)
(Atomic mass: \(\text{Cl = 35.5 amu, H = 1 amu)}\)
1. \(10.2~\text{M}\)
2. \(12.5~\text{M}\)
3. \(14.0~\text{M}\)
4. \(18.2 ~\text{M}\)
Subtopic: Concentration Based Problem |
82%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Two elements \(\mathrm{A}\) and \(\mathrm{B}\) which form \(0.15\) moles of \(\mathrm{A_2B}\) and \(\mathrm{AB_3}\) type compounds. If both \(\mathrm{A_2B}\) and \(\mathrm{AB_3}\) weigh equally, then the atomic weight of A is how many times that of B?
1. 8 times
2. 5 times
3. 3 times
4. 2 times
1. 8 times
2. 5 times
3. 3 times
4. 2 times
Subtopic: Moles, Atoms & Electrons |
83%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
The neutralization occurs when \(10~\text{mL}\) of \(0.1\text{M}\) acid ‘A’ is allowed to react with \(30~\text{mL}\) of \(0.05~\text{M}\) base \(\text{M(OH)}_2.\) The basicity of the acid ‘A’ is:
(M is a metal)
1. 1
2. 2
3. 3
4. 4
(M is a metal)
1. 1
2. 2
3. 3
4. 4
Subtopic: Concentration Based Problem |
84%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Haemoglobin contains \(0.34\%\) of iron by mass. The number of \(\mathrm{Fe}\) atoms in \(\mathrm{3.3~g}\) of haemoglobin is:
\(\small\mathrm{(Given : Atomic ~mass~ of ~Fe~ is~56 \mathrm{u}, \mathrm{N}_{\mathrm{A}}=6.022 \times\left.10^{23} \mathrm{~mol}^{-1}\right)}\)
1. \(1.21 \times 10^5 \)
2. \(12.0 \times 10^{16} \)
3. \(1.21 \times 10^{20} \)
4. \(3.4 \times 10^{22}\)
\(\small\mathrm{(Given : Atomic ~mass~ of ~Fe~ is~56 \mathrm{u}, \mathrm{N}_{\mathrm{A}}=6.022 \times\left.10^{23} \mathrm{~mol}^{-1}\right)}\)
1. \(1.21 \times 10^5 \)
2. \(12.0 \times 10^{16} \)
3. \(1.21 \times 10^{20} \)
4. \(3.4 \times 10^{22}\)
Subtopic: Moles, Atoms & Electrons |
68%
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
\(120~\text{g}\) of an organic compound that contains only carbon and hydrogen gives \( 330~\text{g}\) of \(\text{CO}_2\) and \(270~\text{g}\) of water on complete combustion. The percentage of carbon and hydrogen, respectively are:
1. \(25\) and \(75\)
2. \(40\) and 60
3. 60 and \(40\)
4. \(75\) and \(25\)
1. \(25\) and \(75\)
2. \(40\) and 60
3. 60 and \(40\)
4. \(75\) and \(25\)
Subtopic: Concentration Based Problem |
60%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.






