The correct order with respect to –I effect of the substituents is:
(R = alkyl)
1. –NH2 > –OR < –F
2. –NR2 < –OR < –F
3. –NH2 > –OR > –F
4. –NR2 > –OR > –F
The most stable carbocation among the following is:
| 1. |  ` ` |
2. |  |
| 3. |  |
4. |  |
The IUPAC name of the above mentioned compound is-
1. 3-Ket-2-methylhex-4-enal
2. 5-Formylhex-2-en-3-one
3. 5-Methyl-4-oxohex-2-en-5-al
4. 3-Keto-2-methylhex-4-enal
Which of the following statements is true regarding the conformers of ethane?
| 1. | Bond angle remains the same but bond length changes. |
| 2. | Bond angle changes but bond length remains the same. |
| 3. | Both bond angle and bond length change. |
| 4. | Both bond angle and bond length remain the same. |
The correct order of acidity among the following is:
1. CH2=CH2 >CH≡CH > CH3C≡CH > CH3-CH3
2. CH≡CH > CH3-C≡CH > CH2=CH2 >CH3-CH3
3. CH≡CH > CH2=CH2 > CH3-C≡CH > CH3-CH3
4. CH3-CH3 > CH2=CH2 > CH3-C≡CH > CH≡CH
1. Chromatography
2. Crystallization
3. Steam distillation
4. Sublimation
The IUPAC name of the compound 
1. 5-formylhex-2-en-3-one
2. 5-methyl-4-2-en-5-el
3. 3-keto-2-methylhex-5-enal
4. 3-keto-2-methylhex-4-enal
The correct statement regarding electrophile is :
| 1. | Electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from another electrophile |
| 2. | Electrophiles are generally neutral species and can form a bond by accepting a pair of electrons from a nucleophile |
| 3. | Electrophiles can be either neutral or positively charged species and can form a bond accepting a pair of electrons from a nucleophile |
| 4. | Electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from a nucleophile |
Which among the given molecules can exhibit tautomerisrn?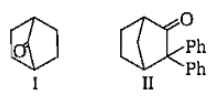
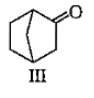
1. III Only
2. Both I and III
3. Both Iand II
4. Both II and III
The correct order of strengths of the carboxylic acids :-
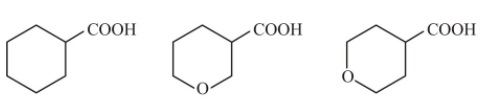
(a) I>II>III
(b) II>III>I
(c) III>II>I
(d) lI>I>IlI







