An incorrect statement among the following:
| 1. | The H-O-H bond angle in H2O is larger than the H-C-H bond angle in CH4 |
| 2. | The H-C-H bond angle in CH4 is larger than the H-N-H bond angle in NH3 |
| 3. | The H-C-H bond angle in CH4, the H-N-H bond angle in NH3 and the H-0-H bond angle in H2O are all greater than 90o |
| 4. | The H-O-H bond angle in H2O is smaller than the H-N-H bond angle in NH3 |
Subtopic: Hybridisation | V.S.E.P.R & V.B.T |
74%
Level 2: 60%+
NEET - 2016
Hints
In piperidine "N" atom has hybridization:
1. sp
2.
3.
4.
Subtopic: Hybridisation |
71%
Level 2: 60%+
Hints
The number of (i) sp2 hybridized carbon atoms and (ii) bonds are present in the following compound are:
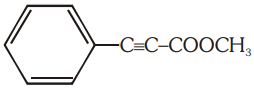
1. 7, 5
2. 8, 6
3. 7, 6
4. 8, 5
Subtopic: Hybridisation |
73%
Level 2: 60%+
Hints
The molecular geometry of XeF5⁺ will be:
1. Square pyramidal
2. Square planar
3. Trigonal bipyramidal
4. Octahedral
1. Square pyramidal
2. Square planar
3. Trigonal bipyramidal
4. Octahedral
Subtopic: Hybridisation |
69%
Level 2: 60%+
Hints
In a regular octahedral molecule, MX6, the number of X–M–X bonds at 180º is:
| 1. | Two | 2. | Six |
| 3. | Four | 4. | Three |
Subtopic: Hybridisation |
73%
Level 2: 60%+
AIPMT - 2004
Hints



