The correct statement regarding the comparison of staggered and eclipsed conformations of ethane is:
| 1. | The eclipsed conformation of ethane is more stable than staggered conformation because eclipsed conformation has no torsional strain. |
| 2. | The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has a torsional strain. |
| 3. | The staggered conformation of ethane is more stable than eclipsed conformation because staggered conformation has no torsional strain. |
| 4. | The staggered conformation of ethane is less stable than eclipsed conformation because staggered conformation has a torsional strain. |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
An unsaturated hydrocarbon 'A' reacts with two molecules of H2 and upon reductive ozonolysis A gives butane-1,4-dial, ethanal, and propanone.
The IUPAC name of A is:
1. 2-Methylocta-2,6-diene
2. 2-Methylocta-1,5-diene
3. 3-Methylocta-2,6-diene
4. 2-Methylocta-1,6-diene

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The most suitable reagent for the following conversion is-
| 1. | Hg2+/ H+, H2O | 2. | Na/liquid NH3 |
| 3. | H2, Pd/C, quinoline | 4. | Zn/HCl |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
Product \(Z\) in the above-mentioned reaction is:
1. \(\mathrm{CH}_3-\left(\mathrm{CH}_2\right)_3-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3\)
2. \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3\)
3. \(\mathrm{CH}_3\left(\mathrm{CH}_2\right)_4-\mathrm{O}-\mathrm{CH}_3\)
4. \(\mathrm{CH}_3 \mathrm{CH}_2-\mathrm{CH}\left(\mathrm{CH}_3\right)-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3 \)

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The correct IUPAC name of the given compound is:
1. 3-Ethyl-4-ethenylheptane
2. 3-Ethyl-4-propylhex-5-ene
3. 3-(1-Ethyl propyl) hex-1-ene
4. 4-Ethyl-3-propylhex-1-ene

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The incorrect IUPAC name among the following is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The isobutyl group among the following is:
| 1. | 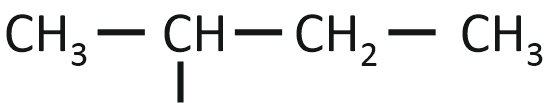 |
| 2. | CH3–CH2–CH2–CH2– |
| 3. | 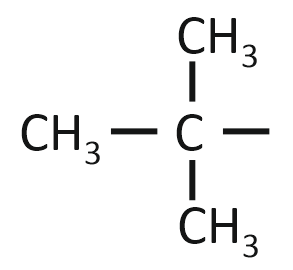 |
| 4. | 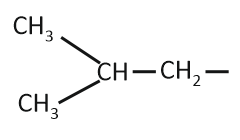 |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
If Compound A (C₄H₈) is treated with H₂O/H₂SO₄ and forms an optically inactive C₄H₁₀O, what is the structure of A?
| 1. | CH3CH2CH=CH2 | 2. | CH3CH=CHCH3 |
| 3. | (CH3)2C=CH2 | 4. |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
A compound having the shortest carbon-carbon bond length among the following is :
1. Benzene
2. Ethene
3. Ethyne
4. Ethane

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
Which compound among the following has the highest boiling point?
| 1. | Iso-octane | 2. | n-Octane |
| 3. | 2,2,3,3-Tetramethyl butane | 4. | n-Butane |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.








