896 mL vapour of a hydrocarbon 'A' having carbon 87.80% and hydrogen 12.19% weighs 3.28g at STP. Hydrogenation of 'A' gives 2-methylpentant. Also 'A' on hydration in the presence of and gives a ketone 'B' having molecular formula . The ketone 'B' gives a positive iodoform test. Find the structure of 'A' and give the reactions involved.
|
Element |
% |
Atomic mass |
Relative ratio |
Relative no. of atoms |
Simplest ratio |
|
C |
87.8 |
12 |
7.31 |
1 |
3 |
|
H |
12.18 |
1 |
12.19 |
1.66 |
4.985 |
Thus, Empirical formula of A is .
Empirical formula mass
Molecular mass is double of empirical formula mass.
Molecular formula is
To determine the structure of compounds (A) and (B)
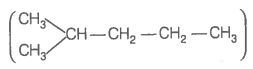
Hence, hydrogenation of hydrocarbon (A) requires 2 moles of hydrogen to form 2-methylpentane. Therefore, hydrocarbon (A) is an alkyne having five carbon atoms in a straight chain and a methyl substituent at position 2. Thus, the possible structures for the alkyne (A) are I and II.
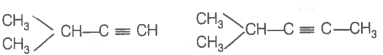
Since, addition of H2O to alkyne (A) in presence of Hg2+, give a ketone which gives positive iodoform test, therefore, ketone (B) must be a methyl ketone, i.e., it must contain a COCH3 group.
Now addition of H2O to alkyne (II) should give a mixture of two ketones in which 2-methylpentan-3 one (minor) and 4-methylpentan-2-one ketone (B) (which shows +ve iodoform test) predominates.
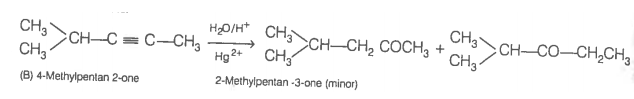
In contrast, addition of H2O to alkyne (I) will give only one ketone, i.e., 4-methylpentan-2-one which gives iodoform test.
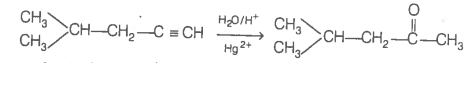
Thus, hydrocarbon is 4-methylpent-1-yne. 4-methylpentan-2 one (gives +ve iodoform test)

© 2026 GoodEd Technologies Pvt. Ltd.