Write the mechanism of the reactioni of HI with methoxybenzene.
Mechanism: Protonation of anisole gives methylphenyl oxonium ion.
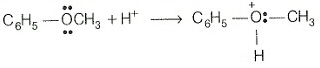
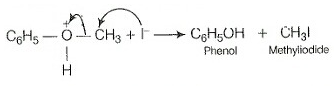
In this ion, the bond between O —CH3, is weaker than the bond between which has partial double bond character. This partial double bond character is due to the resonance between the lone pair of electrons on the O -atom and the hybridised carbon atom of the phenyl group. Therefore, attack by ion exclusively breaks the weaker bond forming methyl iodide and phenol.

© 2026 GoodEd Technologies Pvt. Ltd.