10.18 p-Dichlorobenzene has higher m.p. than those of o- and m-isomers. Discuss.
r 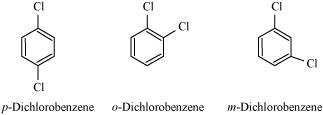
p-Dichlorobenzene is more symmetrical than o-and m-isomers. For this reason, it fits more
closely than o-and m-isomers in the crystal lattice. Therefore, more energy is required to
break the crystal lattice of p-dichlorobenzene. As a result, p-dichlorobenzene has a higher
melting point and lower solubility than o-and m-isomers.

© 2026 GoodEd Technologies Pvt. Ltd.