Which of the following compounds can be classified as aryl halides?
1. p-ClC6H4CH2CH(CH3)2
2. p-CH3CHCl(C6H4)CH2CH3
3. o-BrH2C-C6H4CH(CH3)CH2CH3
4. C6H5-Cl
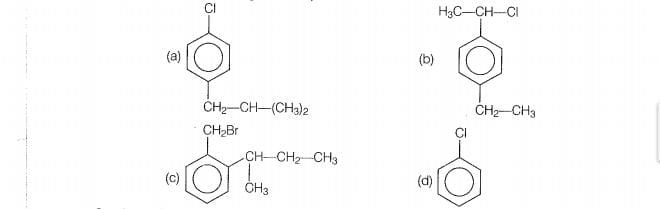
On comparing the above structures it is very obvious that in compound (a) and compound (d),
the halogen atom is directly connected to aromatic ring hence these compounds are divided
as aryl halides.

© 2026 GoodEd Technologies Pvt. Ltd.