Which of the following statements are correct?
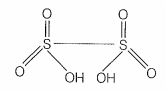
| a. | S — S bond is present in H2S2O6 |
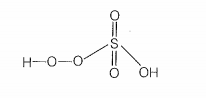
| b. | In peroxosulphuric acid (H2SO5) sulphur is in +6 oxidation state |
| c. | Iron powder along with Al2O3 and K2O is used as a catalyst in the preparation of NH3 by Haber's process |
| d. | Change in enthalpy is positive for the preparation of SO3 by catalytic oxidation of SO2 |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, d)

© 2026 GoodEd Technologies Pvt. Ltd.