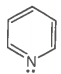
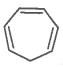
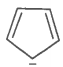
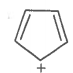
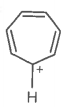
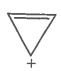
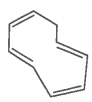
The ring systems having the following characteristics are aromatic.
(a). Planar ring containing conjugated bonds.
(b). Complete delocalisation of the -electrons in the ring system i.e., each atom in the ring has unhybridised p-orbital, and
(c). Presence of -electrons in the ring where n is an integer (n=0,1,2,...) [Huckel rule].
Using this information, the number of aromatic compounds are:
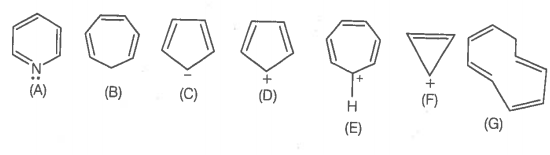
1. 2
2. 4
3. 5
4. 3
|
Compound |
Planar Ring |
Complete delocalization of -electron |
Huckel rule electron |
Aromatic or non-aromatic |
|
|
A. |
|
Yes |
Yes |
Huckel rule obeyed |
Aromatic |
|
B. |
|
No |
No, Incomplete (sp3 hybrid carbon) |
Non-aromatic |
|
|
C. |
|
Yes |
Yes |
(4n+2+lone pair e-) Huckel rule verified |
Aromatic |
|
D. |
|
Yes |
No |
Anti-aromatic |
|
|
E. |
|
Yes |
Yes |
Huckel rule obeyed |
Aromatic |
|
F. |
|
Yes |
Yes |
Huckel rule verified n=0 |
Aromatic |
|
G. |
|
Yes |
No |
Huckel rule not verified |
Non-aromatic |

© 2026 GoodEd Technologies Pvt. Ltd.