Question 12:12:
What are electrophiles and nucleophiles? Explain with exaple.
An electrophile is a reagent that away an electron pair. ln other words, an electron-
seeking reagent is called an electrophile (E) . Electrophiles are electrodeficient and can receive an electron pair.
Carbocations and netrual molecules having functional groups such as carbonyl
group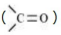 are ecamples of electrophiles.
are ecamples of electrophiles.
A nucleophile is a reagent that brngs an electron pair. ln other words, a nucleus-seeking
For example : OH, NC, carbanions () , etc .
Netural molecules such as and ammonia also act as nuceophilies because of the presence of lone pair.

© 2026 GoodEd Technologies Pvt. Ltd.