7.27 The equilibrium constant for the following reaction is 1.6 ×105 at 1024K
H2(g) + Br2(g) 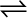 2HBr(g)
2HBr(g)
Find the equilibrium pressure of all gases if 10.0 bar of HBr is introduced into a sealed container at 1024K.
NEET SOLUTION:

© 2026 GoodEd Technologies Pvt. Ltd.