7.15 At 700 K, equilibrium constant for the reaction:
H2 (g) + I2 (g) 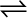 2HI (g)
2HI (g)
is 54.8. If 0.5 mol L–1 of HI(g) is present at equilibrium at 700 K, what are the concentration of H2(g) and I2(g) assuming that we initially started with HI(g) and allowed it to reach equilibrium at 700?

© 2026 GoodEd Technologies Pvt. Ltd.