13.14 Given below are densities of some solids and liquids. Give rough estimates of the size of their atoms :
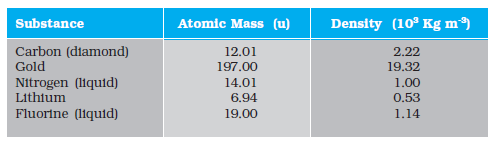
[Hint: Assume the atoms to be ‘tightly packed’ in a solid or liquid phase, and use the known value of Avogadro’s number. You should, however, not take the actual numbers you obtain for various atomic sizes too literally. Because of the crudeness of the tight packing approximation, the results only indicate that atomic sizes are in the range of a few Å].

© 2026 GoodEd Technologies Pvt. Ltd.