The vapour density of undecomposed N2O4 is 46. When heated, vapour density decreases to 24.5 due to its dissociation to NO2. The percent dissociation of N2O4 at the final temperature is:
1. 87
2. 60
3. 40
4. 70
The ratio of the vapour pressure of a solution (containing a non-volatile solute)
to the vapour pressure of the pure solvent is:
1. Equal to the mole fraction of the solvent
2. Proportional to the mole fraction of the solute
3. Equal to the mole fraction of the solute
4. None of the above
The lubricating action of an oil is more if it possess:-
(1) High vapour pressure
(2) low vapour pressure
(3) High surface tension
(4) High density
If partial pressure of oxygen is 0.5 atm and KH = 1.4 × 10-3 M/atm, then the amount of oxygen dissolved in 100 ml water at 298K is-
| 1. | 22.4 mg | 2. | 22.4 g |
| 3. | 2.24 g | 4. | 2.24 mg |
Compound PdCl4.6H2O is a hydrated complex, 1 molal aqueous solution of it has freezing point 269.28 K. Assuming 100 % ionization of complex, the molecular formula of the complex is-
(Kf for water = 1.86 K kg mole-1)
1. [Pd(H2O)6]Cl4
2. [Pd(H2O)4Cl2]Cl2.2H2O
3. [Pd(H2O)3Cl3]Cl.3H2O
4. [Pd(H2O)Cl4]4H2O
How many mili moles of sucrose should be dissolved in 500 gms of water so as to get a solution which has a difference of 103.57°C between boiling point and freezing point.
(K1 = 1.86 K Kg mol−1, Kb = 0.52 K Kg mo1−1)
(1) 500 mmoles
(2) 900 mmoles
(3) 750 mmoles
(4) 650 mmoles
A solution of x moles of sucrose in 100 grams of water freezes at −0.2°C. As ice separated the freezing point goes down to 0.25°C. How many grams of ice would have separated?
1. 10 grams
2. 20 grams
3. 25 grams
4. 23 grams
An ideal mixture of liquids A and B with 2 moles of A and 2 moles of B has a total vapour pressure of 1 atm at a certain temperature. Another mixture with 1 mole of A and 3 moles of B has a vapour pressure greater than 1 atm. But if 4 moles of C are added to the second mixture, the vapour pressure comes down to 1 atm. Vapour pressure of C, Pc° = 0.8 atm. Calculate the vapour pressures of pure A and pure B.
(1) PA° = 1.4 atm, PB° = 0.7 atm
(2) PA = 1.2 atm, PB° = 0.6 atm
(3) PA = 1.4 atm, PB° = 0.6 atm
(4) PA° = 0.6 atm, PB° = 1.4 atm
The vapor pressures of benzene, toluene and a xylene are 75 Torr, 22 Torr and 10 Torr at 20°C. Which of the following is not a possible value of the vapor pressure of an equimolar binary/ternary solution of these at 20°C ? Assume all form ideal solution with each other.
(1) 48
(2) 16
(3)
(4) 53
The molal freezing point constant for water is 1.86°C/m. Therefore, the freezing point of 0.1 m NaCl solution in water is expected to be [MLNR 1994]
(1) –1.86°C
(2) –0.186°C
(3) –0.372°C
(4) +0.372°C
The Van't Hoff factor for 0.1 M solution is 2.74. The degree of dissociation is [IIT 1999]
(1) 91.3%
(2) 87%
(3) 100%
(4) 74%
Two solutions () containing and () containing are separated by semipermeable membrane as shown below. If on reaction with , produces blue colour of , the blue colour will be noticed in: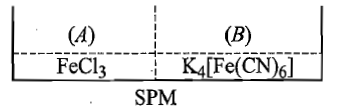
(1) (A)
(2) (B)
(3) in both (A) and (B)
(4) neither in (A) nor in (B)
Each pair forms ideal solution expect:
(1)
(2)
(3)
(4)
The values of observed and calculated molar mass of silver nitrate are 92.64 and 170 respectively. The degree of dissociation of silver nitrate is:
1. 60%
2. 83.5%
3. 46.7%
4. 60.23%
The Henry's law constant for the solubility of N2 gas in water at 298 K is 1.0 atm. The mole fraction of N2 in air is 0.8. The number of moles of
N2 from air dissolved in 10 moles of water at 298 K and 5 atm pressure is:
(1) 4.010-4