If mass of one atom is g, then calculate number of nucleons (neutrons and protons) present in 2 atoms of the element:
1. 40
2. 20
3. 10
4. 40 NA
What is the number of moles of O-atom in 126 amu of :
1.
2.
3.
4.
What is the charge of 96 amu of S2-?
1. 2C
2. 3.210-19C
3. 9.610-19C
4. 6C
The total no. of neutrons present in 54 mL (l) are:
1.
2.
3.
4. none of these
20 g of an ideal gas contains only atoms of S and O occupies 5.6 L at 1 atm and 273 K. What is the mol. wt. of gas?
1. 64
2. 80
3. 96
4. None of these
Total number of moles of oxygen atoms in 3 litre (g) at 27C and 8.21 atm are:
1. 3
2. 4
3. 1
4. none of these
Rearrange the following (I to IV) in the order of increasing masses:
(I) 0.5 mole of
(II) 0.5 gm atom of oxygen
(III) 3.0111023 molecules of
(IV) 5.6 litre of at STP
1. II < IV < III < I
2. II < I < IV < III
3. IV < II < III < I
4. I < II < III < IV
Common salt obtained from sea-water contains 8.775% NaCl by mass. The number of formula units of NaCl present in 25 g of this salt is:
1.
2.
3.
4.
Caffeine has a molecular weight of 194. If it contains 28.9% by mass of nitrogen, number of atoms of nitrogen in one molecule of caffeine is:
1. 4
2. 6
3. 2
4. 3
Density of dry air containing only and is 1.15 g/L at 740 mm and 300 K. What is % composition of by weight in the air?
1. 78%
2. 75.5%
3. 80.5%
4. 72.75%
The vapour density of a mixture containing and is 27.6. The mole fraction of in the mixture is:
1. 0.1
2. 0.2
3. 0.5
4. 0.8
Average atomic mass of magnesium is 24.31 a.m.u. This magnesium is compared of 79 mole % of and remaining 21 mole % of and . Calculate mole of % of .
1. 10
2. 11
3. 15
4. 16
Which of the following law illustrates hydrogen and oxygen combination to form and containing 5.93% and 11.2% hydrogen, respectively?
1. Law of conversation of mass
2. Law of constant proportion
3. Law of reciprocal proportion
4. Law of multiple proportions
One mole of elements X has 0.44 times the mass of one mole of element Y. One atom of elements X has 2.96 times the mass of one atom of . What is the atomic weight of Y?
1. 80
2. 15.77
3. 46.67
4. 40.0
What is the empirical formula of vanadium oxide, if 2.74 g f the metal oxide contains 1.53 g of metal:
1.
2. VO
3.
4.
A 6.85 g sample of the hydrate is dried in an oven to give 3.13 g of anhydrous . What is the value of x? (Atomic weights : Sr=87.60, O=16.0, H=1.0)
1. 8
2. 12
3. 10
4. 6
A gaseous compound is composed of 85.7% by mass carbon and 14.3% by mass hydrogen. It's density is 2.28 g/litre at 300 K and 1.0 atm pressure. Determine the molecular formula of the compound:
1.
2.
3.
4.
What volume of 75% alcohol by weight must be used to prepare of 30% alcohol by weight ?
1.
2.
3.
4. None of these
Nitric acid can be produced in three steps process
(I)
(II)
(III)
percent yield of Ist, IInd and IIIrd are respectiveky 50%, 60% and 80% respectively then what volume of at 1 atm and required to produced 1575 g of .
1. 156.25
2. 350 L
3. 3500 L
4. None of these
M NaOH solution was slowly added into 1000 mL of 183.75 g impure solution and the following plot was obtained. The percentage purity of sample and slope of the curve respectively are:
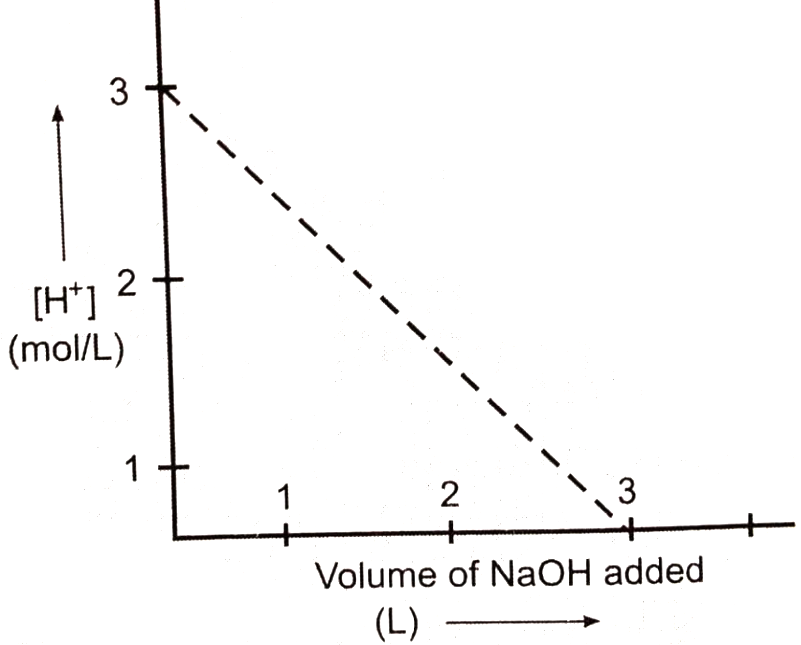
1.
2.
3.
4. None of these
A metal M forms the sulphate . A 0.596 gram sample of the sulphate reacts with excess to give 1.220 g . What is the atomic weight of M? (Atomic weights : S=32, Ba=137.3)
1. 26.9
2. 69.7
3. 55.8
4. 23
The conversion of oxygen to ozone occurs to the extent of 15% only. The mass of ozone that can be prepared from 67.2 L of oxygen at 1 atm and 273 K will be:
1. 14.4 gm
2. 96 gm
3. 640 gm
4. 64 gm