Calculate isothermal compressibility constant for an ideal gas at 2 atm pressure.
1. 0.3 atm-1
2. 0.4 atm-1
3. 0.5 atm-1
4. 0.6 atm-1
A mixture of He and Ar weighting 5.0 g occupies a volume of and 1 atm pressure. The composition of the mixture is respectively -
1. 70%, 30%
2. 25%,75%
3. 75%,25%
4. 40%,60%
An air bubble starts rising from the bottom of a lake, its diameter is 3.6 mm at the bottom and 4 mm at the surface. The depth of the lake is 250 cm and the temperature at the surface is 40C. What is the temperature at the bottom of the lake? (Given atmospheric pressure = 76 cm of Hg and g=980 cm sec-2. Neglect surface tension effect).
1. 293.46 K
2. 193.7K
3. 283.3 K
4. 280.4K
The compression factor (compressibility factor) for one mole of a van der Waal's gas at 0C and 100-atmosphere pressure is found to be 0.5. Assuming that the volume of a gas molecule is negligible, calculate the van der Waals constant 'a'.
1. 0.253
2. 1.253
3.9.998
4.99.32
Calculate Boyle's temperature range for if its van der Waals' constants 'a' and 'b' are 3.592 atm-litre mol2 and b=0.0427 litre mol-1.
1.
2.
3.
4.
The critical constants for water are 374C, 218 atm and 0.0566 litre mol-1. Calculate R.
1. 0.508
2. 0.566
3. 0.0508
4. 0.0566
A 20 g chunk of dry ice is placed in an empty 0.75 litre wine bottle and tightly closed. What would be the final pressure in the bottle after all has been evaporated and temperature reaches to 25C?
1. 14.827 atm
2. 15.827 atm
3. 1.482 atm
4. 1.56 atm
A mixture of and is found to have a density of 1.50 g/litre at 30C and 730 mm. What is the composition of mixture?
1. 40%,60%
2. 22%, 78%
3. 32%,68%
4. 42%,58%
A gas filled freely collapsible balloon is pushed from the surface level of lake to a depth of 100 metre. Calculate what per cent of its original volume the balloon has? Assume ideal gas nature.
1. 10 %
2. 11.4%
3. 9.4%
4. 1.04%
What would be the final pressure of in the following experiment? A collapsed polythene bag of 30 litre capacity is partially blown up by the addition of 10 litre of at 0.965 atm at 298 K. Subsequently enough is pumped into bag so that at 298 K and external pressure of 0.990 atm, the bag contains full 30 litre.
1. 0.99 atm
2. 0.322 atm
3. 0.699 atm
4. 0.668 atm
An evacuated glass vessel weighs 50.0 g when empty, 148.0 g when filled with a liquid of density 0.98 g/mL and 50.5 g when filled with an ideal gas at 760 mm Hg and 300 K. Determine the molar mass of gas.
1. 213 g/mol
2. 312 g/mol
3. 123 g/mol
4. 98 g/mol
For the reaction, , calculate the mole fraction of decomposed at a constant volume and temperature if the initial pressure is 600 mm Hg and the pressure at any time is 960 mm Hg. Assume ideal gas behaviour.
1. 0.3
2. 0.4
3. 0.7
4. 0.6
The compressibility factor for at 273 K and 100 atm pressure is 0.2005. Calculate the volume occupied by 0.2 mole of gas at 100 atm and 273 K using (a) ideal gas nature and (b) real gas nature respectively.
1. 0.044, 0.0089
2. 0.066 , 0.0093
3. 0.022, 0.0045
4. 0.234, 0.0345
8 g He and 20 g Ne molecules both having average velocity are mixed at same temperature. Calculate the kinetic energy/mole of mixture.
1. 979.2 J/mol
2. 879.2 J/mol
3. 840 J / mol
4. 960 J/mol
Positive deviation from ideal behaviour takes place because of:
1. molecular interaction between atoms and PV/nRT > 1
2. molecular interaction between atoms and PV/nRT < 1
3. finite size of atoms and PV/nRT > 1
4. finite size of atoms and PV/nRT < 1
The circulation of blood in human body supplies and releases . The concentration of and is variable but on the average, 100 mL blood contains 0.02 g of and 0.08 g . The volume of and at 1 atm and body temperature 37C, assuming 10 litre blood in human body is:
1. 2 litre, 4 litre
2. 1.5 litre, 4.5 litre
3. 1.59 litre, 4.62 litre
4. 3.82 litre, 4.62 litre
0.014 kg of nitrogen is enclosed in a vessel at 27C. The heat given to gas in order to double its rms speed is:
1. 2.25 kcal
2. 4.50 kcal
3. 1.15 kcal
4. 2 kcal
If are masses of an ideal gas, then which of the graph represents :
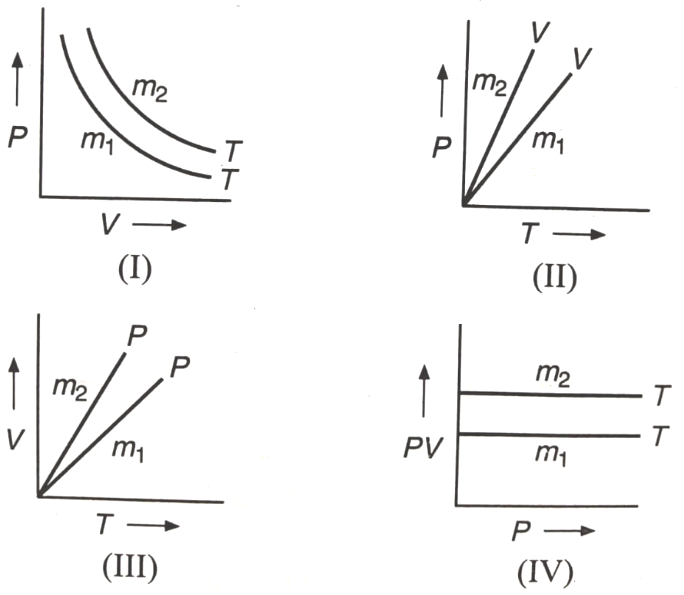
1. I, II only
2. I, III only
3. I, IV only
4. I, II, III, IV
If the intermolecular forces vanish away, the volume occupied by the molecules of 4.5 kg water at STP will be:
1.
2.
3
At sea level air is dense. This is practical application evidence of:
1. Boyle's law
2. Charles' law
3. Dalton's law
4. Avogadro's law
If the limiting slope of density vs. pressure plot for air at is the molar mass of air would be:
1.
2.
3.
4.
The slope for an isochore plotted for a van der Waals' gas neglecting molecular attractions is equal to:
1. zero
2.
3.
4.
A bulb of three litre capacity filled with air is heated from . The air thus, expelled measured 1.45 litre at . Considering the pressure to be 1 atm throughout the experiment and ignoring the expansion of bulb, calculate t.
1. 327 K
2. 427 K
3.
4.
Two containers of equal volume having a sample of gas in are connected through a small tube of negligible volume. what will the relation between final pressure and temperature:
1.
2.
3.
4.
A sample of gas confined in a vessel has some mole at 300 K and 1 atm pressure. Calculate the change in volume when 0.02 mole of gas are added more in the container at 300 K and 1 atm pressure.
1. 0.49 litre
2. 4.09 litre
3. 0.24 litre
4. 2.4 litre