1. The displacement \(x\) of a particle varies with time \(t\) as \(x=ae^{-\alpha t}+be^{\beta t}\) where \(a,b,\alpha\) and \(\beta\) are positive constants. The velocity of the particle will:
1. decrease with time
2. be independent of \(\alpha\) and \(\beta\)
3. drop to zero when \(\alpha=\beta\)
4. increase with time
2.
A transformer having an efficiency of \(75\%\) is working on \(220~\mathrm{V}\) and \(4.4~\mathrm{kW}\) power supply. If the current in the secondary coil is \(5~\mathrm{A}\). What will be the voltage across the secondary coil and the current in the primary coil?
1. \(
V_s=220 \mathrm{~V}, I_p=20 \mathrm{~A}
\)
2. \( V_s=660 \mathrm{~V}, I_p=15 \mathrm{~A}
\)
3. \( V_s=660 \mathrm{~V}, I_p=20 \mathrm{~A}
\)
4. \( V_s=220 \mathrm{~V}, I_p=15 \mathrm{~A}\)
3.
A fish rising vertically to the surface of the water in a lake uniformly at the rate of \(2~\mathrm{m/s}\) observes a kingfisher diving vertically towards the water at a rate of \(10~\mathrm{m/s}\). If the refractive index of water \(n=\frac43\) what will be the actual velocity of the kingfisher?
1. \(
10 \mathrm{~m} / \mathrm{s}
\)
2. \( 8 \mathrm{~m} / \mathrm{s}
\)
3. \( 6 \mathrm{~m} / \mathrm{s}
\)
4. \( 9 \mathrm{~m} / \mathrm{s}\)
4.
One mole of a monoatomic ideal gas undergoes the process \(A\rightarrow B\) in the given \(P-V\) diagram. The molar heat capacity for this process is:

1. \(\frac{3R}{2}\)
2. \(\frac{13R}{6}\)
3. \(\frac{5R}{2}\)
4. \(2R\)
5.
A pan with a set of weights is attached to a light spring. The period of vertical oscillation is \(0.5~\mathrm{s}\). When some additional weights are put in the pan, then the period of oscillations increases by \(0.1\mathrm{~s}\). The extension caused by the additional weight is:
1. \(5.5 \mathrm{~cm}
\)
2. \(3.8 \mathrm{~cm}
\)
3. \(2.7 \mathrm{~cm}
\)
4. \(1.3 \mathrm{~cm}\)
6.
A cylinder of radius \(R\) and length \(L\) is placed in a uniform electric field \(E\) parallel to the cylinder axis. The total flux for the surface of the cylinder is given by:
1. \(2\pi R^2E\)
2. \(\frac{\pi R^2}{E}\)
3. \(\frac RE\)
4. zero
7.
If A + B = C and that C is perpendicular to A. What is the angle between A and B, if |A| = |C|?
1. \(\frac{\pi}{4}~\text{rad}\)
2. \(\frac{\pi}{2}~\text{rad}\)
3. \(\frac{3\pi}{4}~\text{rad}\)
4. \(\pi~\text{rad}\)
8.
A beam of light travelling along X-axis is described by the electric field Ey = 600 V/m. \(\sin~\omega \Big(t-\frac xc\Big),\) the maximum magnetic force on a charge q = 2e, moving along Y-axis with the speed of 3 × 108 m/s is:
1. 19.2 × 10-17 N
2. 1.92 × 10-17 N
3. 0.192 N
4. None of these
9.
A body is whirled in a horizontal circle of radius \(25~\mathrm{cm}\). It has an angular velocity of \(13~\mathrm{rad/s}\). What is its linear velocity at any point on a circular path?
1. \(
2 \mathrm{~m} / \mathrm{s}
\)
2. \( 3 \mathrm{~m} / \mathrm{s}
\)
3. \( 3.25 \mathrm{~m} / \mathrm{s} \)
4. \(
4.25 \mathrm{~m} / \mathrm{s}\)
10.
In the given figure, potential difference between \(A\) and \(B\) is:

1. 0
2. \(5~\mathrm{V}\)
3. \(10~\mathrm{V}\)
4. \(15~\mathrm{V}\)
11.
A block having 12 g of an element is placed in a room. This element is a radioactive element with a half-life of 15 yr. After how many years will there be just 1.5 g of the element in the box?
1. 40 yr
2. 45 yr
3. 20 yr
4. 15 yr
12.
In the given figure what will be the coefficient of mutual inductance?

1. \(\frac{\mu_0a}{2\pi}in\Big(1+\frac{a}{2b}\Big)\)
2. \(\frac{\mu_0a}{\pi}in\Big(1+\frac{b}{2a}\Big)\)
3. \(\frac{\mu_0a}{2\pi}in\Big(1+\frac{a}{b}\Big)\)
4. \(\frac{\mu_0a}{2\pi}in\Big(1+\frac{b}{a}\Big)\)
13.
An \(L-C-R\) series circuit with a resistance of \(100~\Omega\) is connected to \(200~\mathrm{V}\) (AC source) and angular frequency \(300~\mathrm{rad/s}\). When only the capacitor is removed, then the current lags behind the voltage by \(60^\circ\). When only the inductor is removed the current leads the voltage by \(60^\circ\). The average power dissipated in the original \(L-C-R\) circuit is:
1. \(
50 \mathrm{~W}
\)
2. \( 100 \mathrm{~W}
\)
3. \( 200 \mathrm{~W}
\)
4. \( 400 \mathrm{~W}\)
14. The intensity of each of the two slits in Young's double slit experiment is \(I_0\). What is the minimum separation between the points on the screen, where intensities are \(2I_0\) and \(I_0\), if fringe width is \(b\)?
1. \(b \over 5 \)
2. \(b \over 8 \)
3. \(b \over 12 \)
4. \(b \over 4\)
15.
A metal rod at a temperature of \(145^\circ\text{C}\), radiates energy at a rate of \(17~\text{W}\). If its temperature is increased to \(273^\circ\text{C}\), then it will radiate at the rate of:
1. \( 49.6~\text{W} \)
2. \( 17.5~\text{W} \)
3. \( 50.3~\text{W} \)
4. \(67.5~\text{W} \)
16.
Two wires are stretched through the same distance. The force constant of the second wire is half of that of the first wire. The ratio of work done to stretch the first wire and the second wire will be:
1. 2 : 1
2. 1 : 2
3. 3 : 1
4. 1 : 3
17.
A square wire of side \(2.0~\mathrm{cm}\) is placed \(20~\mathrm{cm}\) in front of a concave mirror of focal length \(10~\mathrm{cm}\) with its centre on the axis of the mirror and its plane normal to the axis. The area enclosed by the image of wire is:
1. \(
7.5 \mathrm{~cm}^2
\)
2. \( 6 \mathrm{~cm}^2
\)
3. \(2 \mathrm{~cm}^2 \)
4. \(
4 \mathrm{~cm}^2\)
18.
A lead bullet penetrates into a solid and melts. Assuming that \(50\%\) of its kinetic energy was used to heat it, the initial speed of the bullet is: (the initial temperature of the bullet is \(25^\circ\mathrm{C}
\) and its melting point is \(300^\circ\mathrm{C}
\)). Latent heat of fusion of lead \(=2.5\times10^4~\mathrm{J/Kg}\) and specific heat capacity of lead \(=125~\mathrm{J/Kg-K}\).
1. \(
100 \mathrm{~m} / \mathrm{s}
\)
2. \( 490 \mathrm{~m} / \mathrm{s}
\)
3. \( 520 \mathrm{~m} / \mathrm{s}
\)
4. \( 360 \mathrm{~m} / \mathrm{s}\)
19.
A quarter cylinder of radius
\(R\) and refractive index
\(1.5\) is placed on a table. A point object
\(P\) is kept at a distance of
\(mR\) from it as shown in the figure. For what value of
\(m\) for which a ray from
\(P\) will emerge parallel to the table?

1.
\(
1 / 2
\)
2.
\( 1 / 3
\)
3.
\( 4 / 3
\)
4.
\( 2 / 3\)
20.
The graph
\(\frac{1}{\lambda}\) and stopping potential (
\(V\)) of three metals having work function
\(\phi_1,~\phi_2\) and
\(\phi_3\) in an experiment of the photoelectric effect is plotted as shown in the figure. Which one of the following statements is/are correct? [Here A. Is the wavelength of the incident ray]

| (i) |
Ratio of work functions \(\phi_1,\phi_2\) and \(\phi_3\) = 1: 2: 4 |
| (ii) |
Ratio of work functions \(\phi_1,\phi_2\) and \(\phi_3\) = 4: 2:1 |
| (iii) |
\(\tan\theta\propto\frac{hc}{e},\) Where h = plank’s constant, c = speed of light |
| (iv) |
The violet-colour light can eject photoelectrons from metals 2 and 3 |
1. (i), (iii)
2. (i), (iv)
3. (ii), (iii)
4. (i), (ii) and (iv)
21.
The concentric, conducting spherical shells \(X,~ Y\) and \(Z\) with radii \(r, ~2r\) and \(3r\) respectively. \(X\) and \(Z\) are connected by a conducting wire and \(Y\) is uniformly charged to charge \(Q\) as shown in the figure. Charges on shells \(X\) and \(Z\) will be:

1. \(qx=\frac{Q}{4},qz=\frac{-Q}{6}\)
2. \(qx=\frac{-Q}{4},qz=\frac{Q}{4}\)
3. \(qx=\frac{Q}{4},qz=\frac{-Q}{4}\)
4. \(qx=\frac{-Q}{6},qz=\frac{Q}{4}\)
22.
The temperature of a gas is raised from \(27^\circ\mathrm{C}\) to \(927^\circ\mathrm{C}\). The root mean square speed:
| 1. |
gets halved |
| 2. |
gets doubled |
| 3. |
is \(\Big(\sqrt{\Big(\frac{927}{27}}\Big)\Big)\) times the earlier value |
| 4. |
remains the same |
23.
A particle slides down on a smooth incline of inclination , fixed in an elevator going up with an acceleration of 2 m/s2. The box of incline has a length of 4 m. The time taken by the particle to reach the bottom will be:

1. \(\frac89\sqrt3s\)
2. \(\frac98\sqrt3s\)
3. \(\frac43\sqrt{\frac{\sqrt3}{2}}s\)
4. \(\frac34\sqrt{\frac{\sqrt3}{2}}s\)
24.
A stone projected with a velocity \(u\) at an angle \(\Big(\frac{\pi}{2}-\theta\Big)\) with the horizontal reaches maximum height \(H_1\) When it is projected with velocity \(u\) at an angle with the horizontal, it reaches maximum height Hz. The relation between the horizontal range \(R\) of the projectile, \(H_1\) and \(H_2\) is:
1. \(R=4\sqrt{H_1H_2}\)
2. \(R=4({H_1-H_2})\)
3. \(R=4({H_1+H_2})\)
4. \(R=\frac{H^2_1}{H_2^2}\)
25.
Two batteries of emf \(3~\text V\) and \(6~\text V\) with internal resistances \(2~\Omega\) and \(4~\Omega\) are connected in a circuit with a resistance of \(10~\Omega\) as shown in the figure. The current and potential difference between the points \(P\) and \(Q\) are:

1. \(\frac{3}{16}A~\text{and}~\frac{8}{15}V\)
2. \(\frac{16}{3}A~\text{and}~\frac{15}{8}V\)
3. \(\frac{3}{16}A~\text{and}~8~V\)
4. \(\frac{3}{16}A~\text{and}~\frac{15}{8}V\)
26. A light string passes over a frictionless pulley. To one of its ends, a mass of 8 kg is attached. To its other end, two masses of 7 kg each are attached. The acceleration of the system will be:

1. 10.2 g
2. 5.10 g
3. 20.36 g
4. 0.27 g
27.
A capillary tube of length \(L\) and radius \(v\) is connected with another capillary tube of the same length but half the radius in series. The rate of the steady volume flow of water through the first capillary tube under a pressure difference of \(p\) is \(V.\) The rate of the steady volume flow through the combination will be: (the pressure difference across the combination is \(p\))
1. \(17~V\)
2. \(\frac{16}{17}~V\)
3. \(\frac{V}{17}\)
4. \(\frac{17}{16}~V\)
28.
A system consists of a cylinder surrounded by a cylindrical shell. A cylinder has a radius \(R\) and is made of material of thermal conductivity \(K,\) whereas a cylindrical shell has an inner radius \(R\) and outer radius \(2R\) and is made of material of thermal conductivity twice as that of a cylinder. Assuming the system is in a steady state and has negligible heat loss across the cylindrical surface, what is the effective thermal conductivity of the system if the two ends of the combined system are maintained at two different temperatures?
1. \(3K\)
2. \(\frac23K\)
3. \(\frac{7K}{4}\)
4. \(\frac{5K}{4}\)
29.
An isotropic point source emits light with wavelength 500 nm. The radiation power of the source is P = 10 W. The number of photons passing through a unit area per second at a distance of 3 m from the source is:
1. 5.92 × 1017 m/s2
2. 2.23 × 1017 m/s2
3. 2.23 × 1018 m/s2
4. 5.92 × 1018 m/s2
30.
If a proton and antiproton come close to each other and annihilate, how much energy will be released?
1. \( 1.5 \times 10^{-10} \mathrm{~J} \)
2. \( 3 \times 10^{-10} \mathrm{~J} \)
3. \( 4.5 \times 10^{-10} \mathrm{~J} \)
4. \( 2 \times 10^{-10} \mathrm{~J}\)
31.
A body at rest slides down a 30° inclined plane. The time taken by it to slide down is twice the time it takes when it slides down the same distance in the absence of friction. The coefficient of friction between the body and the inclined plane is:
1. 0.43
2. 0.37
3. 0.64
4. 0.75
32.
A bat emitting an ultrasonic wave of frequency 4.5 × 104 Hz at speed of 6 m/s between two parallel walls. The two frequencies heard by the bat will be:
1. 4.67 × 104 Hz, 4.34 × 104 Hz
2. 4.34 × 104 Hz, 4.67 × 104 Hz
3. 4.5 × 104 Hz, 5.4 × 104 Hz
4. 4.67 × 103 Hz, 4.34 × 104 Hz
33. The charges on two spheres are \(+7~\mu C\) and \(-5~\mu C\) respectively. They experience a force \(F.\) If each of them is given an additional charge of \(-2~\mu C,\) then the new force of attraction will be:
1. \(F\)
2. \(\frac{F}{2}\)
3. \(\frac{F}{\sqrt3}\)
4. \(2F\)
34.
A rod made up of metal is 1.2 m long and 0.8 cm in diameter. Its resistance is 3.5 × 10-3 \(\Omega.\) Another disc made of the same metal is 2.0 cm in diameter and 1.25 mm thick. What is the resistance between the round faces of the disc?
1. 1.35 × 10-8 \(\Omega\)
2. 2.70 × 10-7 \(\Omega\)
3. 5.82 × 10-7 \(\Omega\)
4. 8.10 × 10-5 \(\Omega\)
35.
A body is moving along a rough horizontal surface with an initial velocity of 10 ms-1 If the body comes to rest after travelling a distance of 12 m, then the coefficient of sliding friction will be:
1. 0.5
2. 0.2
3. 0.4
4. 0.6
36.
The magnification produced by an astronomical telescope for normal adjustment is \(10\) and the length of the telescope is \(1.1~\mathrm{m}\). The magnification, when the image is formed at least distance of distinct vision is:
1. \(6\)
2. \(18\)
3. \(16\)
4. \(14\)
37.
A circular current-carrying coil has a radius \(R.\) The distance from the centre of the coil, on the axis, where \(B\) will be \(\frac18\) of its value at the centre of the coil is:
1. \(\frac{R}{\sqrt3}\)
2. \(\sqrt3R\)
3. \(2\sqrt3R\)
4. \(\frac{2R}{\sqrt3}\)
38.
Angular width of central maximum in the Fraunhofer diffraction pattern of a slit is measured. The slit is illuminated by light of wavelength \(6000~\mathring{\mathrm{A}}\). When the slit is illuminated by light of another wavelength, then the angular width decreases by \(30\%\). The same decrease in angular width of the central maximum is obtained when the original apparatus is immersed in a liquid. The refractive index of the liquid will be:
1. \(
1.25
\)
2. \( 1.42
\)
3. \( 1.67
\)
4. \( 1.5\)
39.
An energy of \(68.0~\mathrm{eV}\) is required to excite a hydrogen-like atom in its second Bohr energy level to third energy level the charge of a nucleus is The wavelength of radiation required to eject the electron from first orbit to infinity is:
1. \(2.2 \mathrm{~nm} \)
2. \(2.85 \mathrm{~nm} \)
3. \(3.2 \mathrm{~nm} \)
4. \(2.5 \mathrm{~nm}\)
40.
A current carrying loop is placed in a uniform magnetic field in four different orientations
\(\mathrm{I,II,III}\) and
\(\mathrm{IV}\) as shown in the figure. Arrange them in decreasing order of potential energy.

1.
\( \mathrm{ I>I I I>I I>I V } \)
2.
\(\mathrm{ I>I I>I I I>I V }\)
3.
\( \mathrm{ I>I V>I I>I I I }\)
4.
\(\mathrm{I I I > I V>I>I I}\)
41.
Two different isotherms representing the relationship between pressure \(P\) and volume \(V\) at a given temperature of the same ideal gas are shown for masses \(m_1\) and \(m_2,\) then:

| 1. |
nothing can be predicted |
| 2. |
\(m_1 < m_2\) |
| 3. |
\(m_1 = m_2\) |
| 4. |
\(m_1 > m_2\) |
42.
A 7Li target is bombarded with a proton beam current 10-4 A for one hour to produce 7Be of activity 1.8 × 108 disintegrations per second. Assuming that one 7Be radioactive nuclei is produced by bombarding 1000 protons, its half-life is:
1. 0.87 × 107 s
2. 0.2 × 107 s
3. 0.67 × 108 s
4. 0.87 × 106 s
43.
In the given figure, the capacitors C1, C3, C4, C5 have a capacitance \(4~\mu F\) each. If the capacitor C2 has a capacitance of \(10~\mu F,\) then the effective capacitance between \(A\) and \(B\) will be:

1. \(2~\mu F\)
2. \(6~\mu F\)
3. \(4~\mu F\)
4. \(8~\mu F\)
44.
The truth table for the following logic circuit is:

| 1. |
0 |
0 |
0 |
| 0 |
1 |
1 |
| 1 |
0 |
1 |
| 1 |
1 |
0 |
| 2. |
0 |
0 |
1 |
| 0 |
1 |
1 |
| 1 |
0 |
1 |
| 1 |
1 |
1 |
| 3. |
0 |
0 |
1 |
| 0 |
1 |
0 |
| 1 |
0 |
1 |
| 1 |
1 |
0 |
| 4. |
0 |
0 |
1 |
| 0 |
1 |
1 |
| 1 |
0 |
0 |
| 1 |
1 |
1 |
45.
A sphere of mass m moving with velocity \(v\) hits in elastically with another stationary sphere of the same mass. The ratio of their final velocities will be: (in terms of \(e\))
1. \(\frac{v_1}{v_2}=\frac{1+e}{1-e}\)
2. \(\frac{v_1}{v_2}=\frac{1-e}{1+e}\)
3. \(\frac{v_1}{v_2}=\frac{1+e}{2}\)
4. \(\frac{v_1}{v_2}=\frac{1-e}{2}\)
46.
A small spherical drop falls from rest in a viscous liquid. Due to friction, heat is produced. The correct relation between the rate of production of heat and the radius of the spherical drop at terminal velocity will be:
1. \(\frac{dH}{dT}\propto\frac{1}{r^5}\)
2. \(\frac{dH}{dT}\propto r^4\)
3. \(\frac{dH}{dT}\propto\frac{1}{r^4}\)
4. \(\frac{dH}{dT}\propto r^5\)
47.
A galvanometer of resistance 25 \(\Omega\) shows a deflection of 5 divisions when a current of 2 mA is passed through it. If a shunt of 4 \(\Omega\) is connected and there are 20 divisions on the scale, then the range of the galvanometer is:
1. 1 A
2. 58 A
3. 58 mA
4. 30 mA
48.
The total charge induced in a conducting loop when it is moved in magnetic field depends on:
1. The rate of change of magnetic flux
2. Initial magnetic flux only
3. The total change in magnetic flux
4. Final magnetic flux only
49.
Particles of masses \(m,2m.3m,\dots,nm\) are placed on the same line at distances \(L,2L,3L,\dots,nL\) from \(O\). The distance of the centre of mass from \(O\) is:
1. \(\frac{(2n+1)L}{4}\)
2. \(\frac{L}{(2n+1)L}\)
3. \(\frac{n(n^2+1)L}{2}\)
4. \(\frac{(2n+1)L}{3}\)
50.
The length of a given cylindrical wire is increased by 150%. Due to the consequent decrease in diameter, the change in the resistance of the wire will be:
1. 200%
2. 525%
3. 300%
4. 400%
51.
An ideal solenoid having 5000 turns/m has an aluminium core and carries a current of 5 A. If \(\chi_{_{Al}}=2.3\times10^{-5},\) then the magnetic field developed at the centre will be:
1. 0.031 T
2. 0.048 T
3. 0.027 T
4. 0.050 T
52.
A ball of radius R rolls without slipping. Find the fraction of total energy associated with its rotational energy, if the radius of the gyration of the ball about an axis passing through its centre of mass is K.
1. \(\frac{K^2}{K^2+R^2}\)
2. \(\frac{R^2}{K^2+R^2}\)
3. \(\frac{K^2+R^2}{R^2}\)
4. \(\frac{K^2}{R^2}\)
53.
A capacitor is charged and then made to discharge through a resistance. The time constant is \(\tau.\) In what time will the potential difference across the capacitor decrease by 10%?
1. \(\tau \ln 0.1\)
2. \(\tau \ln 0.9\)
3. \(\tau \ln \frac{10}{9}\)
4. \(\tau \ln \frac{11}{10}\)
54.
A body of mass 2 m is placed on earth's surface. Calculate the change in gravitational potential energy, if this body is taken from earth's surface to a height of \(h,\) where \(h = 4R.\)
1. \(\frac{2mgh}{R}\)
2. \(\frac23mgR\)
3. \(\frac85mgR\)
4. \(\frac{mgR}{2}\)
55.
The slope of isothermal and adiabatic curves are related as:
| 1. |
Isothermal curve slope = adiabatic curve slope |
| 2. |
Isothermal curve slope = \(\gamma\) × adiabatic curve slope |
| 3. |
Adiabatic curve slope = \(\gamma\) × isothermal curve slope |
| 4. |
Adiabatic curve slope = \(\frac12\) × isothermal curve slope |
56.
A solid sphere of mass M and radius 2R rolls down an inclined plane of height h without slipping. The speed of its centre of mass when it reaches the bottom is:
1. \(\sqrt{\frac{6}{7} g h}\)
2. \(\sqrt{3 g h}\)
3. \(\sqrt{\frac{10}{7} g h}\)
4. \(\sqrt{\frac{4}{3} g h}\)
57. A liquid of density \(800~\text{kg/m}^3\) is filled in a cylindrical vessel up to a height of \(3~\text{m}\). This cylindrical vessel stands on a horizontal plane. There is a circular hole on the side of the vessel. What should be the minimum diameter of the hole to move the vessel on the floor, if the plug is removed. Take the coefficient of friction between the bottom of the vessel and the plane as \(0.5\) and the total mass of the vessel plus vessel as \(95~\text{kg}\).
1. \(0.107~\text{m}\)
2. \(0.053~\text{m}\)
3. \(0.206~\text{m}\)
4. \(0.535~\text{m}\)
58.
The transfer ratio \(\beta\) of a transistor is 50. The input resistance of the transistor when used in the common emitter configuration is 2 k\(\Omega.\) The peak value of the collector AC current for an AC input voltage of 0.02 V peak is:
1. 200 \(\mu\)A
2. 0.01 mA
3. 0.25 mA
4. 500 \(\mu\)A
59.
The volume of an ideal gas is doubled in an isothermal process. Then, which of the following is true?
| 1. |
Work done by the gas is positive. |
| 2. |
Work done by the gas is negative. |
| 3. |
Internal energy of the system decreases. |
| 4. |
Internal energy of the system increases. |
60.
A prism of a certain angle deviates the red and blue rays by 8 and 12, respectively. Another prism of the same angle deviates the red and blue rays by 10 and 14, respectively. The prisms are small angled and made of different materials. The dispersive power of the materials of the prisms is in the ratio:
1. 5 : 6
2. 9 : 11
3. 6 : 5
4. 11 : 9
61.
Temperature of a gas is t K. What would be the temperature at which volume and pressure, both will reduced to half of the initial values?
1. t/2
2. t/4
3. t/3
4. t/8
62.
What will be the number of waves formed by a Bohr electron in one complete revolution in its second orbit?
1. Three
2. Two
3. One
4. Zero
63.
When 2-iodo-2-methylpentane is treated with sodium ethoxide in ethanol,
the major product will be:
1. 2-Methylbut-2-ene and carbon dioxide
2. 2-Methylpent-1-ene
3. 2-Methyl pent-2-ene
4. 2-Methylpentane
64. The weight of solid NaHCO3 (in grams) required to neutralize 40.0 mL of 0.1 M H2SO4 solution is:
1. 0.672 g
2. 6.07 g
3. 17 g
4. 20 g
65.
The pair of boiling point and compound are given as,

Which will show the lowest vapour pressure at room temperature?
1. C
6H
6
2. CH
3OH
3. C
6H
5NO
2
4. C
6H
5NH
2
67.
Ethers like ROR can be cleaved by concentrated HI but not by HCl because
68.
In the given reaction sequence:
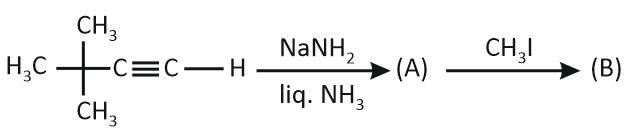
How many electron-donating groups are attached to the carbon atom of the unsaturated part of the product 'B'?
1. Two
2. Three
3. Four
4. None of the above
70.
Which of the following hydrocarbons is the most reactive towards addition of H2S04?
1. Ethane
2. Propylene
3. 3-methyl but-1-ene
4. 1-butene
71.
Choose the incorrect statement about noble gas?
1. Boiling point increases with increasing atomic mass
2. Helium has least tendency to form compound
3. Noble gases has some value of electron affinity
4. Xenon has maximum number of compounds
72. Given the cell reaction \(Pb+Sn^{2+}~\longrightarrow~Pb^{2+}+Sn\) . Also, given that, \(Pb~\longrightarrow~Pb^{2+},E^0=0.13~V\) and \(Sn^{2+}+2e^-~\longrightarrow~Sn;E^0=-0.14~V\). What would be the ratio of log of concentration of cation of lead to the conc of cation of tin for which \(E = 0?\)
1. 1/4
2. 1/2
3. 1/3
4. 1/1
73.
[CuCl4]2- exists while [Cul4]2- does not exist, because:
1. I- is a stronger reductant than Cl-
2. I- is a weaker reductant than Cl-
3. I- is a stronger oxidant than Cl-
4. None of the above.
76.
Actinides exhibit larger number of oxidation state than that of corresponding lanthanides. The reason behind this aspect is
1. Lesser energy difference between 5f and 6d-orbitals than between 4f and 5d-orbitals
2. Larger atomic size of actinides than the lanthanides
3. More energy difference between 5f and 6d orbitals than between 4f and 5d-orbitals
4. Greater reactive nature of the actinides than the lanthanides
78.
The melting point of solid substances is directly proportional to pressure acting on them. However, ice-melts at a temperature lower than its usual melting point, when the pressure increases. This is because
1. Ice is not a true solid
2. The bonds break under pressure
3. Ice is loss denser than water
4. Pressure generates heat
79.
The Eo values for Mn and Zn are more negative than expected because
1. They have either half-filled or fully-filled configurations
2. They can easily donate electrons
3. It is quite easy to remove electrons from their orbitals
4. None of the above
81.
Work is being performed, when a weightlifter lifts a baseball off a weight rack. This is due to
1. Magnetic attraction
2. Gravity
3. Electrostatic repulsion
4. None of these
83.
Beryllium differs in properties from other elements of its own group but shows resemblance with aluminium because of
1. Relatively bigger ionic radius and high polarising power of be
2. Relatively smaller ionic radius and high polarising power of be
3. Relatively bigger ionic radius is the only reason behind this
4. None of the above
84.
Two similar reactions have the same rate constant at 25 °C, but at 35 °C, one of the reaction has a higher rate constant than the other. The appropriate reason for this is
1. Due to effective collisions
2. Due to different activation energies
3. Due to different threshold energies
4. Due to higher population of molecules
86.
Actinides exhibit larger number of oxidation state than that of corresponding lanthanides. The reason behind this aspect is
1. Lesser energy difference between 5f and 6d-orbitals than between 4f and 5d-orbitals
2. Larger atomic size of actinides than the lanthanides
3. More energy difference between 5f and 6d orbitals than between 4f and 5d-orbitals
4. Greater reactive nature of the actinides than the lanthanides
89.
Lauryl alcohol on treatment with H2SO4 followed by neutralisation forms a product which is
1. Cationic detergent
2. Anionic detergent
3. Neutral detergent
4. Anti-depressant
90.
When an iron object is plated with tin, tin does not act as sacrificial anode in protecting against corrosion, because.
1. Tin is more reactive than iron
2. Tin is less reactive than iron
3. Reactivity of tin and iron is same
4. tin is oxidising agent while iron is not so
94.
100 mL of a solution contains 2g of acetic acid and 3g of sodium acetate providing Ka = 1.8 x 10-5, then choose the correct Option.
1. This solution is basic in nature
2. This solution is acidic in nature
3. This solution is amphoteric in nature
4. This solution is neutral in nature
95.
Consider the following structures.

Choose the correct statement regarding the above structures.
1. Dipole moment varies as II > III > I
2. II is more stable than I
3. I is the most reactive among three
4. All of the above
96.
On the basis of Langmuir adsorption isotherm the amount of gas adsorbed at very high pressure.
1 .Reaches a constant limiting value
2. Goes on increasing with pressure
3. Goes on decreasing with pressure
4. First increasing and then decreasing with pressure
98.
Consider the following reaction sequence

Choose the correct option regarding the different products obtained in the above reaction sequence.
1. '0' is an alcohol consisting one carbon more than the starting alcohol
2. Product '8 is formed via SN2-pathway
3. 'C' is an amine
4. All of the above
101.
Consider the following compounds.

The decreasing order of decarboxylation is
1. I> II> III
2. III> II>I
3. III > I > II
4. II > I > III
103.
C3H9N reacts with Hinsberg reagent and the product is insoluble in alkali but soluble in ether. This nitrogen containing compound is
1. Primary amine
2. Secondary amine
3. Tertiary amine
4. Methyl isocyanides
104.

Product ‘Q’ is
1. An amide
2. An amine
3. Nitro compound
4. Nitrile compound
105.
Which of the following statements is incorrect?
1. Polypropylene is a thermoplastic polymer
2. Melamine-formaldehyde is a thermosetting Polymer
3. Mixture of styrene and methyl methacrylate can Form ionic addition polymer
4. Low-density polythene is a poor conductor of electricity
106.
Match the particle with its characteristic.
Column I
A. α - particle
B. Isobar
C. γ - ray
D. β - particle
Column II
(p) Slow moving
(q) High penetration power
(r) Same atomic mass
(s) Consists of electron
1. A-p, B-r, C-q, D-s
2. A-p, B-q, C-r, D-s
3. A-r, B-s, C-p, D-q
4. A-s, B-r, C-p, D-q
107.
The magnitude of screening effect depends upon the number of
1. Inner electrons
2. outer electrons
3. Bond order
4. Both A and B
109.
Sugars are separated by using the solvent BAW (n-butanol acetic acid -H20) and detected by spraying the plate with
1. Aniline hydrogen phthalate solution
2. Hydrogen peroxide solution
3. Crystals of 12
4. Cupric oxide
112.
At radioactive equilibrium, the ratio between two atoms of radioactive elements A and B is 3.1x 109:1. If the half-life period of A is 2 x 1010 years then the half-life of B is
1. 9.54 yrs.
2. 2.14 yrs.
3. 3.29 yrs.
4. 6.45 yrs.
120.
The value of n in

is
1. 5
2. 4
3. 4
4. 3
121.
Consider following statements and Choose correct ones from given options.
I. Shark does not have any bone in its body.
II. Water snake and salamander belongs to same class and have largest RBC.
III. Silver fish is a true fish while cuttle and star fishes are molluscs and echinoderms respectively.
IV. Ornithorhynchus is a connecting link
Between reptiles and mammals.
1. I, II and IV
2. I and IV
3. I, II and III
4. Ill and IV
122.
Which one of the following is not correctly matched?
1. Diphtheria corynebacterium
2. Elephantiasis wuchereria
3. Plague paramyxo
4. Lockjaw clostridium
123.
The largest muscle in the human is
1. Biceps
2. Gluteus Maximus
3. Stapedius
4. masseter
124.
The best description of natural selection is
1. The reproductive success of the members of a population best adapted to environment
2. It acts when the resources are unlimited
3. A change in the proportion of variations within a population
4. It follows Hardy-Weinberg principle
126. Adenosine triphosphate was discovered by
1. Jack Lipman
2. A Bloor
3. Karl Lohmann
4. Emil Fisher
127.
Lens of eyes is derived from
1. Ectoderm
2. Mesoderm
3. Endoderm
4. Both B and C
128.
Schneiderian and tympanic membranes respectively belongs to
1. Ear and nose
2. ear and eye
3. Ear and ear
4. nose and ear
130.
Who received Nobel Prize in 2008 for the discovery of HIV?
1. Harald Zur Hausen
2. Luc Montagnier
3. Jack Szostak
4. Carol Greider
131.
Which one of the following is a recognisation site for restriction enzyme Bam HIV?
1. 5'- GAATIC- 3'3'- CTIAAG- 5'
2. 5'- GGATCC- 3'5'- CCATGG- 3'
3. 5'- GGATCC- 3'3'- CCTAGG- 5'
4. 5'- GAATIC- 3'5', - GTIAAC- 3'
132.
Golden ages of reptiles and fishes are respectively
1. Mesozoic and Devonian
2. Jurassic and Permian
3. Triassic and Silurian
4. Palaeozoic and Mesozoic
133.
Match the following Columns.

1. A-2, B-4, C-1, D-3
2. A-2, B-3, C-4, D-1
3. A-3, B-4, C-1, D-2
4. A-3, B-1, C-4, D-2
134.
Which one of following pair is not correctly matched?
1. Almoner - Drupe
2. Brinjal- Berry
3. Guava- Pepo
4. Loquat- Pome
135.
The powerhouse of cell is first discovered by
1. C Benda in 1897
2. Kolliker in 1850
3. Claude in 1880
4. Kingsburg in 1882
136.
During chloride shift or Hamburger phenomenon, when the whole blood is saturated with C02, following changes occurs. Which one of them is not correct?
1. Bicarbonate content of plasma and corpuscles increase
2. Chloride content of plasma diminished and that of the cell is increased
3. Total base of blood remain unchanged
4. Water content and volume of corpuscles decrease
138.
Common phase in aerobic and anaerobic Respiration is
1. Krebs' cycle
2. Glycolysis
3. Glycogenolysis
4. ETS
139.
Treponema pallidum' pathogen is a cause of
1. Leprosy
2. plague
3. Syphilis
4. pertussis
140.
Which one of the following pair is not correct?
1. Mangolian idiocy - 21st chromosome
2. Patau syndrome-13th chromosome
3. Cri-Du-Chat- 11th chromosome
4. Edward syndrome -18th chromosome
141.
Bacteriophage

is different from other bacteriophage due to the presence of
1. Single-stranded DNA
2. Single stranded RNA
3. double-stranded DNA
4. None of the above
142.
An irregular mode of reproduction resulting in the development of an embryo without fertilisation is called
I. parthenogenesis
II. Apogamy
III. Sporophytic budding
Select the answer using the code correct given below.
1. Only I
2. Only II
3. II and III
4. I, II and III
143.
In which one of the family formations of endosperm doesn't take place?
1. Orchidaceae
2. Cactaceae
3. Ranunculaceae
4. Malvaceae
144.
In cardiac cycle maximum time is taken by
1. Atria systole
2. Atria diastole
3. Ventricle systole
4. Ventricle diastole
145.
A horse and a donkey can breed to produce mule which is an infertile animal. The infertility is because horse and donkey belong to different
1. Class
2. order
3. Species
4. genus
146.
Source of commercial chewing gum latex is
1. Hevea brasiliensis
2. Carica papaya
3. Ficus elastica
4. Achras sapota
147.
Compare the statement I and II and choose the correct option.
Statement I:In the flowering plants due to higher accumulation of auxins dormancy of lateral buds occurs.
Statement II: In Maryland Mammoth (a tobacco variety) flowering occurred at a different time at different latitude due to gibberellin concentration.
1. Statement I is true, but II is false
2. Statement I is false, but II is true
3. Both statements are true
4. Both statements are false
148.
Which one of the given pollination technique/adaptation is different than others?
1. Herkogamy
2. Geitonogamy
3. Dichogamy
4. Heterostyly
149.
The phenotypic ratio of trihybrid crosses in F2 - generation is
1. 27 : 9 : 9 : 9 : 3 : 3 : 3 : 1
2. 9: 3: 3: 1
3. 1: 4: 6: 4: 1
4. 27 : 9: 3: 3: 9: 1: 2: 1
150.
A point mutation where guanine is replaced by cytosine is also called
1. frame shift mutation
2. Transition mutation
3. Translocation mutation
4. Transversion mutation
151.
Match the following Columns.

1. A-4, B-3, C-2, D-1
2. A-2, B-3, C-1, D-4
3. A-3, B-1, C-2, D-4
4. A-4, B-2, C-1, D-3
152.
Match the following Columns.

1. A-1 B-4 C-2 D-3
2. A-3 B-4 C-1 D-2
3. A-1 B-3 C-2 D-4
4. A-3 B-1 C-4 D-2
153.
Which one of the following antibodies plays an important role as mediator in allergic response?
1. lg E
2. lg G
3. lg D
4. lg A
154.
Which one of the following human ancestors is known as tool maker?
1. Homo erectus
2. Java man
3. Homo habilis
4. Heidelberg man
155.
Which one of the following is a matching pair of vector and the disease?
1. Culex- Filariasis
2. Housefly- Leprosy
3. Aedes aegypti- Chickenpox
4. Sandfly- Cholera
156.
Consider these following (Sentences and choose the correct ones.
I. Each gene contains a specific promoter region and a leader sequence for guiding the beginning of transcription.
II. Only one strand of DNA called the template strand is copied by RNA polymerase this strand runs in 3'→5'direction.
III. RNA polymerase adds complementary nucleotides forming single strand mRNA in 3'→5' direction.
IV. Section of DNA that has been transcribed is rewound into its original configuration.
1. II, III and IV
2. II and IV
3. I, II and IV
4. I, ll, and IV
157.
Which one of the following is not correct pair of type of cancer and origin place?
1. Benign tumour- Non-cancerous tumour
2. Carcinomas - Cancer of epithelial tissues
3. Lymphomas - Haematopoietic cells tumour
4. Sarcomas -Cancer of gland (secretory tissues)
158.
In animals normally which organism has maximum number of chromosomes?
1. Butterfly
2. Elephant
3. Hermit crab
4. Chimpanzee
159.
During meiosis-I, the bivalent chromosomes clearly appear as tetrads during.
1. Diakinesis
2. Diplotene
3. Pachytene
4. zygotene.
160.
Snapdragon flower is an exception of Mendel's laws. It is a good example of
1. Law of dominance
2. Complementary gene
3. Co-dominance
4. incomplete-Dominance
162.
The term 'prebiotic soup' for organic water containing mixture of simple organic compounds was given by
1. Richter
2. Haldane
3. Arrhenius
4. Miller
163.
Number of Barr body which will found in case of Turner's syndrome will be
1. 1
2. 2
3. 0
4. Can't be determine by given data
164.
Which one of the following options is not a sexually transmitted disease?
1. AIDS
2. Hepatitis-B
3. Pertussis
4. Syphilis
165.
The Sub-units of 80S ribosome will be
1. 40S, 40S
2. 60S, 40S
3. 60S, 20S
4. 55S, 25S
166.
Pyloric sphincter guards the opening between
1. Stomach and duodenum
2. Cardiac and fundus
3. Oesophagus and stomach
4. Fundus and pylorus
167.
Vegetative propagation in Bryophyllum takes place through
1. Bulbil
2. corms
3. Leaf buds
4. eyes
168.
Pappus helps in dispersal of seed in
1. Asteraceae
2. Brassicaceae
3. Malvaceae
4. Solanaceae
169.
Diphtheria is caused by
1. Poison released by living bacterial cell into the host
2. Poison released from dead bacterial cell into the host
3. Poison released by virus into the host
4. Excessive immune response by the body of host
170.
Which one of the following microbes is the source for vitamin-C?
1. Pseudomonas sp.
2. Acetobacter sp.
3. Aspergillus sp.
4. Chlorella
171.
The fact that DNA is a genetic material was established by the experiment of
1. Meselson and Stahl
2. Hershey and Chase
3. Avery, Macleod and McCarty
4. Rosalind Franklin and Kornberg
172.
Match the following Columns.

1. A-3, B-2, C-1, D-4
2. A-2, B-4, C-1, D-3
3. A-4, B-3, C-2, D-1
4. A-1, B-3, C-2, D-4
173.
Which one of the following hormones is released by posterior lobe of pituitary gland?
1. FSH
2. ADH
3. ACTH
4. MSH
174.
During the transmission of nerve impulse through a nerve fibre, the potential on the inner side of the plasma membrane has which type of electric charge?
1. First negative then positive and again back to negative
2. First positive then negative and continue to be negative
3. First negative then positive and continue to be positive
4. First positive then negative and again back to positive
175.
Uric acid is the excretory waste of
1. Adult amphibians
2. Birds
3. Amphibian larvae
4. Mammals
176.
In case of pregnancy the heartbeat of embryo starts at
1. 4th week
2. 7th week
3. 6th week
4. 5th week
177.
PPLO is smallest cell in the living world.
The extend form of PPLO is
1. Pseudo Pneumonia Length Orge
2. Pseudo Plank Leg Organelle
3. Pneumonia Plank like Organism
4 . Pleura Pneumonia like Organism
178.
Slime-mould belongs to
1. kingdom-Protista
2. kingdom-Monera
3. Kingdom-Fungi
4. kingdom-Plantae
179.
Which one of the following is not a flower?
1. Shoe-flower
2. Sunflower
3. Larkspur
4. Water lily
180.
A light string passes over a frictionless pulley. To one of its ends a mass of 8 kg is attached. To its other end two masses of 7 kg each are attached. The acceleration of the system will be

1. 10.2 g
2. 5.10 g
3. 20.36 g
4. 0.27 g
*If above link doesn't work, please go to test link from where you got the pdf and fill OMR from there
CLICK HERE to get FREE ACCESS for 2 days of ANY NEETprep course


















