Consider the composite system, which is held at 300 K, shown in the following
figure. Assuming ideal gas behavior, calculate the total pressure if the barriers separating the compartments are removed. Assume that the volume of the barriers is negligible (Given : R = 0.082 atm L/mol. K)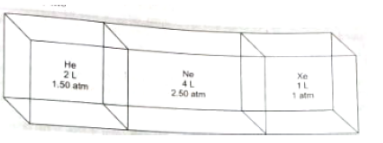
1. 1 atm
2. 2 atm
3. 2.3 atm
4. 3.2 atm
11 moles and 12 moles of mixture reacted in 20 litre vessel at 800 K. After
equilibrium was reached, 6 mole of was present. 3.58 litre of liquid water is
injected in equilibrium mixture and resultant gaseous mixture suddenly cooled to 300 K. What is the final pressure of gaseous mixture ? Neglect vapour pressure of liquid solution. Assume (i) all dissolved in water (ii) no change in volume of liquid (iii) no reaction of and at 300 K
(1) 18.47 atm
(2) 60 atm
(3) 22.5 atm
(4) 45 atm
Two vessels connected by a valve of negligible volume. One container (l) has
2.8 g of at temperature (K). The other container (ll) is completely
evacuated . The container (l) is heated to (K) while container (ll) is maintained at (K), volume of vessel (l) is half that of vessel (ll). If the valve is opened then what is the weight ratio of in both vessel ()?
1. 1:2
2. 1:3
3. 1:6
4. 3:1
A balloon containing 1 mole air at 1 atm initially is filled further with air till
pressure increased to 4 atm. The initial diameter of the balloon is 1 m and the
pressure at each stage is proportional to diameter of the balloon. How many
no. of moles of air added to change the pressure from 1 atm to 4 atm.
1. 80
2. 257
3. 255
4. 256
If Pd v/s P (where P denotes pressure in atm and d denotes density in gm/L) is
plotted for He gas (assume ideal) at a particular temperature. If 5, then the temperature will be
1. 160 K
2. 320 K
3. 80 K
4. None of these
Gas molecules each mass kg are taken in a container of volume 1 .
The root mean square speed of gas molecules is 1 km . What ¡s the
temperature of gas molecules. (Given = 6 X ; = 8 J/mol K)
1. 298 K
2. 25 K
3. 250 K
4. 2500 K
A balloon of diameter 21 meter weight loo kg. Calculate its pay load, if it is
filled with He at 1.0 atm and 27°C. Density of air is 1.2 kg . (given : R =
0.082 L atm )
1. 4952.42 kg
2. 4932.42 kg
3. 493.242 kg
4. None of these
If 250 ml of over water at 30 and a total pressure of 780 torr, what will be
the total pressure if the mixture is ¡n a 500 mL vessel over water at 35 °c {Given: Vapour pressure (Aqueous tension) of at 25 °C, 30°C and 35 °C are 23.8, 31.8 and 42.2 torr respectively. Assume volume of H20 (I) is negligible in final vessel)
1. 760 torr
2. 828.4 torr
3. 807.6 torr
4. 870.6 torr
A mixture of nitrogen and water vapours is admitted to a flask at 760 torr which contains a sufficient solid drying agent after long time the pressure reached a steady value of 722 torr. If the experiment is done at 27°C and drying agent increases in weight by 0.9 gm, what is the volume of the flask? Neglect any possible vapour pressure of drying agent and volume occupied by drying agent.
1. 443.34 L
2. 246.3 L
3. 12.315 L
4. 24.63 L
The density of vapour of a substance (X) at 1 atm pressure and 500 K is 0.8
kg/. The vapour effuses through a small hole at a rate of 4/5 times slover
than oxygen under the same condition. What is the compressibility factor (Z) of
the vapour?
1. 0.974
2. 1.35
3. 1.52
4. 1.22
Vander Waal’s gas equation can be reduced to vinal equation and vinal
equation (in terms of volume) is Z = + ... where A = first vinal
coefficient of Hg(g) is 625 . What volume is available for movement of
10 moles He(g) atoms present in 50 L vessel?
1. 49.75 L
2. 49.25 L
3. 25 L
4. 50 L
If the slope of ‘Z’ (compressibility factor) v/s ‘P’ curve is constant
at a particular temperature (300 K) and very high
pressure, then calculate diameter of the molecules. (Given: =6 X , R=
0.0821 atm lit )
1. 7.5 A°
2. 5 A°
3. 2.5 A°
4. 1.25 A°
Match the items of columns l and ll
| Column - l | Column - ll | ||
| (P) | Z for ideal gas behaviour | (1) | |
| (Q) | Z for real gas at low temperature | (2) | |
| (R) | Z for real gas at high pressure | (3) | 1 |
| (S) | Z for critical rate | (4) |
Codes
P Q R S
1. 1 2 4 3
2. 3 4 2 1
3. 2 1 4 3
4. 1 2 3 4
| List-1 (condtions for real gas) | List -ll | ||
| (P) | If force of attraction among gas particles are negligible | (1) | =RT |
| (Q) | At 1 atm and 273 K | (2) | =RT- |
| (R) | If the volume of gas particles is negligible | (3) | |
| (S) | At low pressure and high temperature | (4) |
P Q R S
1. 4 1 3 2
2. 4 3 2 1
3. 2 1 4 3
4. 1 2 3 4