At what temperature will the rms speed of oxygen molecules become just sufficient for escaping from the Earth's atmosphere (Given: Mass of an oxygen molecule (m) = 2.76x kg, Boltzmann's constant = 1.38x J/K)
1. 2.508 x K
2. 8.360 x K
3. 1.254 x K
4. 5.016 K
A gas mixture consists of 2 moles of and 4 moles of Ar temperature T. Neglecting all vibrational modes, the total internal energy of the system is
1. 4 RT
2. 15 RT
3. 9 RT
4. 11 RT
A given sample of an ideal gas occupies a volume V at a pressure P and absolute temperature T. The mass of each molecule of the gas is m. Which of the following gives the density of the gas?
1.
2.
3.
4. mkT
The molecules of a given mass of a as have r.m.s velocity of 200 at 27°C and 1.0 x pressure. When the temperature and pressure of the gas are respectively 127°C and 0.05 x 105 Nm2, the r.m.s velocity of its molecules in is
1.
2.
3.
4.
Two vessels separately contain two ideal gases A and B at the same temperature, the pressure of A being twice that of B. Under such conditions, the density of A is found to be 1.5 times the density of B. The ratio of molecular weight of A and B
1. 1/2
2. 2/3
3. 3/4
4. 2
The ratio of the specific heats in terms of degrees of freedom (n) is given by
1.
2.
3.
4.
The mean free path of molecules of a gas, (radius r) is inversely proportional to
1.
2.
3. r
4.
The amount of heat energy required to raise the temperature of 1 g of Helium at NTP, from is
1.
2.
3.
4.
An ideal gas is enclosed in a container of volume V at a pressure P. It is being pumped out of the container by using a pump with stroke volume v. What is the final pressure in a container after the n-stroke of the pump? (assume temperature remains same)
1.
2.
3.
4.
A container contains 32g of at a temperature T. The pressure of the gas is P. An identical container containing 4 g of at a temperature 2T has a pressure of
1. 8P
2. 4P
3. P
4.
An ideal gas is expanding such that PT = constant. The coefficient of volume expansion of the gas is
1.
2.
3.
4.
The pressure versus temperature graph of an ideal gas is as shown in the figure. The density of the gas at point A is . Density at point B will be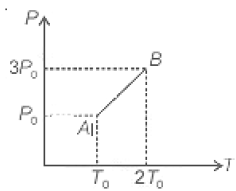
1.
2.
3.
4. 2
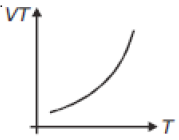
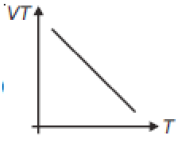
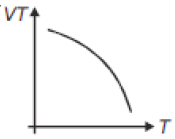
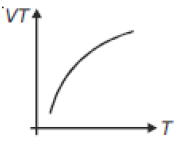
If heat energy is given to an ideal gas at constant pressure, then select hte graph which best represents the variation of VT with temperature (T).
1. 
2. 
3. 
4. 
The temperature (T) of one mole of an ideal gas varies with its volume (V) as , where a and ß are positive constants. The maximum pressure of the gas during this process is
1.
2.
3.
4.
Nitrogen gas is filled in an insulated container. If fraction of moles dissociates without the exchange of any energy, then the fractional change in its temperature is
1.
2.
3.
4.
Nitrogen gas N2 of mass 28 g is kept in a vessel at a pressure of 10 atm and temperature 57°C. Due to leakage of gas, its pressure falls to 5 atm and temperature to 27°C. The amount of gas leaked out is
1.
2.
3.
4.
Three perfect gases at absolute temperatures are mixed. If the number of molecules of the gases are respectively then the temperature of the mixture will be (assume no loss of energy)
1.
2.
3.
4.
The temperature of a gas is - . At what temperature will the average kinetic energy of its molecules be twice that of ?
1.
2.
3.
4.
The ratio of the average translatory kinetic energy of He gas molecules to gas molecules is
1.
2.
3.
4. 1