The normal boiling point of water 373 K. Vapour pressure of water at temperature T is 19 mm Hg. If enthalpy of vaporization is 40.67 kJ/mol, then temperature T would be
(Use : log 2 = 0.3, R = 8.3 ) :
1. 250 K
2. 291.4 K
3. 230 K
4. 290 K
A sample of liquid of mass 18.0 g is injected into an evacuated 7.6 L flask maintained at 27.0C. If vapour pressure of at 27C is 24.63 mm Hg. What weight percentage of the water will be vapourised when the system comes to equilibrium? Assume water vapours behave as an ideal gas. The volume occupied by the liquid water is negligible compared to the volume of the container :
1. 1%
2. 10%
3. 18%
4. 20%
The equal weight of a solute is dissolved in an equal weight of two solvents A and B to form a very dilute solution. The relative lowering of vapour pressure for solution B has twice the relative lowering of vapour pressure for solution A.
If and are the molecular weights of solvents A and B respectively, then:
1. =
2. = 2
3. = 4
4. = 2
The azeotropic mixture of water (B.P = 100 °C) and HCI (B.P = 86 °C) boils at about 120 °C. During fractional distillation of this mixture, it is possible to obtain
1. Pure HCl
2. Pure
3. Pure as well as pure HCl
4. Neither nor HCl
Calculate the percentage degree of dissociation of an electrolyte (Normal molar mass = 164) in water if the observed molar mass by measuring elevation in boiling point is 65.6:
1. 75%
2. 25%
3. 65%
4. None of these
The temperature of a city was found to be -9.3 °C. A car was used, whose radiator was filled with 5 L of water. What quantity of anti-freezing agent ethylene glycol was added to the water radiator in order to use the car for traveling? ( of water 1.86 K kg )
1. 3200 g
2. 1670 g
3. 1550 g
4. 2100 g
0.1 molal aqueous solution of an electrolyte is 90% ionized. The boiling point of the solution at 1 atm is: (=0.52 K kg )
1. 273.19 K
2. 374.92 K
3. 376.4K
4. 373.19 K
Insulin is dissolved in a suitable solvent and the osmotic pressure (T) of solutions of various concentrations (g/) C is measured at 20°C. The slope of a plot n against C is found to be 4.65 x . The molecular weight of the insulin (g/mol) is :
1. 3x
2. 9x
3. 4.5x
4. 5.16x
At 300 K, 40 mL of (g) dissolved in 100 g of water at 1.0 atm. What mass of ozone dissolved in 400 g of water at a pressure of 4.0 atm at 300 K?
1. 0.1 g
2. 1.2 g
3. 0.48 g
4. 4.8 g
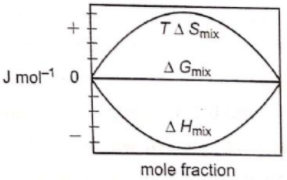
Which of the following represents correctly the changes in the thermodynamic properties during the formation of 1 mole of an ideal binary solution :
1. 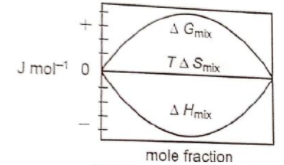
2. 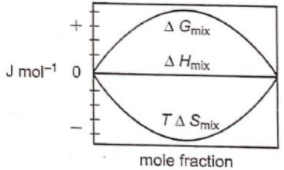
3. 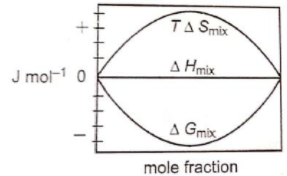
4. 
A saturated solution of has a vapor pressure of 17.20 mm Hg at 20°C, while pure water vapor pressure is 17.25 mm Hg. Solubility product () of at 20°C
1. 9.8x
2.
3. 2.56x
4. 7x
A certain non-volatile electrolyte contains 40% carbon, 6.7% hydrogen, and 53.3% oxygen. An aqueous solution containing 5% by mass of the solute boils at 100.15°C. The molecular formula of the compound is:
( = 0.51°C/m)
1. HCHO
2.
3.
4.
0.1 M KI and 0.2 M are mixe in a 3:1 volume ratio. The depression of the freezing point of the resulting solution will be [ = 1.86 K kg ]
1. 3.72 K
2. 1.86 K
3. 0.93 K
4. 0.279 K.
It is known that the atom contains protons, neutrons, and electrons. If the mass of a neutron is assumed to half to its original value whereas that of the proton is assumed to be twice of its original value then the atomic mass of will be :
1. Same
2. 14.28% less
3. 14.28 % more
4. 28.56%
The density of dry air containing only and is 1.15 g/L at 740 mm and 300 K. What is % composition of by weight in the air:
1. 78%
2. 75.5%
3. 70.08%
4. 72.25%
What is the empirical formula of vanadium oxide, 2.74 g of the metal oxide contains 1.5 g of metal?
1.
2. VO
3.
4.
The hydrated salt undergoes 63% loss in mass on heating and becomes anhydrous.
The value of x is:
1. 10
2. 12
3. 8
4. 18
A gaseous compound is composed of 85.7% by mass carbon and 14.3% by mass hydrogen. Its density is 2.28 g/liter at 300 K and 1.0 atm pressure. Determine the molecular formula of the compound:
1.
2.
3.
4.
2.0 g sample contains a mixture of SiO2 and Fe2O3, on very strong heating leave a residue weighing 1.96 g. The reaction responsible for the loss of weight is (unbalanced equation) What is the percentage by mass of SiO2 in the original sample?
1. 10%
2. 20%
3. 40%
4. 60%
An ideal gaseous mixture of ethane () and ethene () occupies 28 litres at 1 atm and 273 K. The mixture reacts completely with 128 g to produce and . Mole fraction at in the mixture is:
1. 0.6
2. 0.4
3. 0.5
4. 0.8
2 Mole of loses 16 moles of the electron is being converted to a new compound X. Assuming that all of the N appears in the new compound. What is the oxidation state of 'N' in X?
1. -1
2. -2
3. +2
4. +4
One litre of a sample of hard water contains 4.44 mg and 1.9 mg of . Water is the total hardness in terms of ppm of ?
1. 2 ppm
2. 3 ppm
3. 4 ppm
4. 6 ppm