A small bubble rises from the bottom of a lake, where the temperature and pressure are 8C and 6.0 atm, to the water's surface, where the temperature is 25C and pressure is 1.0 atm. Calculate the final volume of the bubble if its initial volume was 2 mL.
1. 14 mL
2. 12.72 mL
3. 11.31 mL
4. 15 mL
Starting out on a trip into the mountains, you inflate the tires on your automobile to a recommended pressure of 3.21 x 10 Pa on a day when the temperature is -5.0C. You drive to the beach, where the temperature is 28.0C. Assume that the volume of the tire has increased by 3%. What is the final pressure in the tires?
1. 350 Pa
2. 3500 Pa
3. 3.5 x 10 Pa
4. None of these
The intercept on y-axis and slope of curve plotted between P/T vs. T.
For an ideal gas having 10 moles in a closed rigid container of volume 8.21 L. (P = Pressure is atm and T = Temp. in K, log2 = 0.30) are respectively :
1. 0.01, 0
2. 0.1, 0
3. 0.1, 1
4. 10, 1
The density of gas A is twice that to B at the same temperature the molecular weight of gas B is twice that of A. The ratio of pressure of gas A and B will be :
1. 1 : 6
2. 1 : 1
3. 4 : 1
4. 1 : 4
An open flask containing air is heated from 300 K to 500 K. What percentage of air will be escaped to the atmosphere, if pressure is keeping constant?
1. 80
2. 40
3. 60
4. 20
A manometer attached to a flask contains with ammonia gas have no difference in MERCURY level initially as shown in diagram. After sparking into the flask, ammonia is partially dissociated as now it have difference of 6 cm in mercury level in two columns, what is partial pressure of at equilibrium?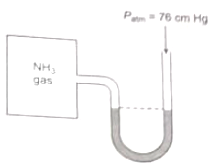
1. 9 cm Hg 2. 18 cm Hg
3. 27 cm Hg 4. None of these
Two closed vessel A and B of equal volume of 8.21 L are connected by a narrow tube of negligible volume with open valve. The left hand side container is found to contain 3 mole CO and 2 mole of He at 400 K, what is the partial pressure of He in vessel B at 500 K?
1. 2.4 atm
2. 8 atm
3. 12 atm
4. None of these
At STP, a container has 1 mole of He, 2 moles Ne, 3 moles of , and 4 moles . Without changing total pressure if 2 moles of is removed, the partial pressure of will be decreased by-
1. 26 %
2. 40 %
3. 58.33 %
4. 66.66 %
A 821 mL N (g) was collected over liquid water at 300 K and 1 atm. if vapour pressure of HO is 30 torr then moles of N (g) in moist gas mixture is :
1. 0.39
2. 0.032
3. 0.96
4. 0.0013
The vapour pressure of water at 80C is 355 mm of Hg. 1 L vessel contains at 80C, saturated with water the total pressure being 760 mm of Hg. The contents of the vessel were pumped into 0.3 L vessel at the same temperature. What is the partial pressure of ?
1. 1350 Hg
2. 2178.3 Hg
3. 121.5 Hg
4. 355 Hg
A jar contains a gas and a few drops of water. The pressure in the jar is 830 mm of Hg. The temperature of the jar is reduced by 1%. The vapour pressure of water at two temperature are 30 and 25 mm of Hg. Calculate the new pressure in jar.
1. 792 mm of Hg
2. 817 mm of Hg
3. 800 mm of Hg
4. 840 mm of Hg
A gaseous mixture three gases A, B and C with a total number of moles of 10 and total pressure of 10 atm. The partial pressure A and B are 3 atm and 1 atm respectively and if C has molecular weight of 2 g/mol. Then, the weight of C in the mixture will be :
1. 8 g
2. 12 g
3. 3 g
4. 6 g
A rigid container containing 5 mole gas at same pressure and temperature. The gas has been allowed to escape by simple process from the container due to which pressure of the gas becomes half of its intial pressure and temperature become (2/3)rd of its initial. The mass of gas remaining is :
1. 7.5 g 2. 1.5 g
3. 2.5 g 4. 3.5 g
Oxygen gas generated by the decomposition of potassium chlorate is collected over water. The volume of oxygen collected at 24C and atmospheric pressure of 760 mm Hg is 128 mL. Calculate the mass of oxygen gas obtained. The pressure of the water vapour at 24C is 22.4 mm Hg
1. 1.36 g
2. 1.52 g
3. 0.163 g
4. 1.63 g
Two flask A and B of equal volumes maintained 300 k and 700 k contain equal mass of He(g) and N2(g) respectively. What is the ratio of translational kinetic energy of gas in flask A to that of flask B ?
1. 1:3
2. 3:1
3. 3:49
4. None of these
Calculate relative rate of effusion of through a container in 3:2 mass ratio
1.
2.
3.
4. None of these
80 mL of takes 2 minute to pass through the hole. What volume of will pass through the hole in 3 minute ?
1.
2.
3.
4. None of these
Dimethyl ether decomposes as
When decomposes to 20% extent at certain fixed conditions, what is the ratio of diffusion of pure with methane ?
1. 0.59 :1
2. 1.18 :1
3. 2.36 :1
4. 1.77 : 1
4 gm of sulphur dioxide gas diffuses from a container in 8 min. Mass of helium gas diffusing from the same container over the same time interval is :
1. 0.5 gm
2. 1 gm
3. 2 gm
4. None of these