Physics
1. In Young's-double slit experiment, the distance between the slits and the screen is doubled. The separation between the slits is reduced to half. As a result the fringe width:
| 1. |
is halved |
| 2. |
become four times |
| 3. |
remains unchanged |
| 4. |
is doubled |
2. Two rods of equal length
\(1~\text{m}\) and equal cross-sectional area
\(0.01~\text{cm}^2\) are joined in series, as shown. One rod is copper with resistivity
\(1.7\times10^{-6}~\Omega\text{-cm},\) and the other is iron with resistivity
\(10^{-5}~\Omega\text-\text{cm}.\) What voltage must be applied across the combination to maintain a current of
\(1~\text A\) in the circuit?
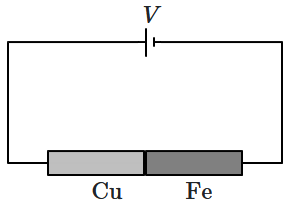
| 1. |
\(0.00145~\text V\) |
2. |
\(0.0145~\text V\) |
| 3. |
\(1.7\times10^{-6}~\text V\) |
4. |
\(0.117~\text V\) |
3. A long straight wire carries a certain current and produces a magnetic field
\(2 \times 10^{-4} ~\text{Wb/m}^2\) at a perpendicular distance of
\(5~\text{cm}\) from the wire. An electron situated at
\(5~\text{cm}\) from the wire moves with a velocity
\(10^7 ~\text{m/s}\) towards the wire along perpendicular to it. The force experienced by the electron will be:
(The charge on electron =
\(1.6 \times 10^{-19} ~\text C\) )
| 1. |
\(3.2~\text N\) |
2. |
\(3.2 \times 10^{-16} ~\text N\) |
| 3. |
\(1.6 \times 10^{-16} ~\text N\) |
4. |
zero |
4. The output from a
\(\mathrm{NAND}\) gate is divided into two in parallel and fed to another
\(\mathrm{NAND}\) gate. The resulting gate is a:

1.
\(\mathrm{AND}\) gate
2.
\(\mathrm{NOR}\) gate
3.
\(\mathrm{OR}\) gate
4.
\(\mathrm{NOT}\) gate
5. The ratio of radii of gyration of a circular ring and a circular disc, of the same mass and radius, about an axis passing through their centres and perpendicular to their planes are:
1. \(1 : \sqrt 2\)
2. \(3:2\)
3. \(2:1\)
4. \( \sqrt 2 : 1 \)
6. The pair of quantities having the same dimensions are:
| 1. |
impulse and surface tension |
| 2. |
angular momentum and work |
| 3. |
work and torque |
| 4. |
Young's modulus and energy |
7. A system is taken from state
\(a\) to state
\(c\) by two paths
\(adc\) and
\(abc\) as shown in the figure. The internal energy at
\(a\) is
\({U}_{a}=10~\text{J}.\) Along the path
\(adc\) the amount of heat absorbed
\(\delta Q_1=50~\text{J}\) and the work obtained
\(\delta W_1=20~\text{J}\) whereas along the path
\(abc\) the heat absorbed
\(\delta Q_2 = 36~\text{J}.\) The amount of work along the path
\(abc\) is:

| 1. |
\(10~\text{J}\) |
2. |
\(12~\text{J}\) |
| 3. |
\(36~\text{J}\) |
4. |
\(6~\text{J}\) |
8. The primary coil of a transformer, when connected to a
\(10~\text{V}\text-\)DC battery, draws a current of
\(1~\text{mA}.\) The number of turns in the primary and secondary coils are
\(50\) and
\(100,\) respectively. The voltage across the secondary coil and the current drawn by the circuit in the secondary are respectively:
| 1. |
\(20~\text{V}\) and \(2.0~\text{mA}\) |
| 2. |
\(10~\text{V}\) and \(0.5~\text{mA}\) |
| 3. |
zero and therefore no current |
| 4. |
\(20~\text{V}\) and \(0.5~\text{mA}\) |
9. How does the binding energy per nucleon vary with the increase in the number of nucleons?
| 1. |
decrease continuously with mass number. |
| 2. |
first decreases and then increases with an increase in mass number. |
| 3. |
first increases and then decreases with an increase in mass number. |
| 4. |
increases continuously with mass number. |
10. The length of a wire between the two ends of a sonometer is
\(100~\text{cm}.\) What should be the positions of two bridges below the wire so that the three segments of the wire have their fundamental frequencies in the ratio
\(1:3:5\)?
| 1. |
\(\dfrac{1500}{23} \mathrm{~cm}, \dfrac{500}{23} \mathrm{~cm} \) |
| 2. |
\(\dfrac{1500}{23} \mathrm{~cm}, \dfrac{300}{23} \mathrm{~cm} \) |
| 3. |
\(\dfrac{300}{23} \mathrm{~cm}, \dfrac{1500}{23} \mathrm{~cm} \) |
| 4. |
\(\dfrac{1500}{23} \mathrm{~cm}, \dfrac{2000}{23} \mathrm{~cm}\) |
11. An electric dipole of dipole moment \({p}\) is aligned parallel to a uniform electric field \({E}.\) The energy required to rotate the dipole by \(90^\circ\) is:
1. \( p^2 E\)
2. \( p E\)
3. infinite
4. \( {pE}^2\)
12. A charge
\(q\) is placed at the centre of the line joining two equal positive charges
\(Q.\) The system of the three charges will be in equilibrium, if
\(q\) is equal to:
| 1. |
\(\dfrac{-Q}{4}\) |
2. |
\(\dfrac{Q}{4}\) |
| 3. |
\(\dfrac{-Q}{2}\) |
4. |
\(\dfrac{Q}{2}\) |
13. A car is moving in a circular horizontal track of radius
\(10~\text m\) with a constant speed of
\(10~\text{m/s}.\) A bob is suspended from the roof of the car by a light wire of length
\(1.0~\text m.\) The angle made by the wire with the vertical is:
| 1. |
\(\dfrac{\pi}{3} \) |
2. |
\(\dfrac{\pi}{6}\) |
| 3. |
\(\dfrac{\pi}{4}\) |
4. |
\(0^\circ\) |
14. A bar magnet of the magnetic moment
\(M\) is placed at right angles to a magnetic induction
\(B.\) If a force
\(F\) is experienced by each pole of the magnet, the length of the magnet will be:
| 1. |
\(\dfrac{MB}{F}\) |
2. |
\(\dfrac{BF}{M}\) |
| 3. |
\(\dfrac{MF}{B}\) |
4. |
\(\dfrac{F}{MB}\) |
15. The radius of a planet is twice the radius of the Earth. Both have almost equal average mass densities. If
\(v_P\) and
\(v_E\) are escape velocities of the planet and the earth, respectively, then:
| 1. |
\(v_P = 1.5 v_E\) |
2. |
\(v_P = 2v_E\) |
| 3. |
\(v_E = 3 v_P\) |
4. |
\(v_E = 1.5v_P\) |
16. If the ratio of diameters, lengths and Young's modulus of steel and copper wires shown in the figure are
\(p,\) \(q\) and
\(s\) respectively, then the corresponding ratio of increase in their lengths would be:

| 1. |
\(\dfrac{5 q}{\left(7 {sp}^2\right)} \) |
2. |
\(\dfrac{7 q}{\left(5 sp^2\right)} \) |
| 3. |
\(\dfrac{2 q}{(5 s p)} \) |
4. |
\(\dfrac{7 q}{(5 s p)}\) |
17. A parallel beam of light of wavelength \(\lambda\) is incident normally on a narrow slit. A diffraction pattern is formed on a screen placed perpendicular to the direction of the incident beam. At the second minimum of the diffraction pattern, the phase difference between the rays coming from the two edges of the slit is:
1. \(2 \pi\)
2. \(3 \pi\)
3. \(4 \pi\)
4. \( \pi \lambda\)
18. A particle of mass
\(m\) is kept at rest at a height
\(3R\) from the surface of the Earth, where
\(R\) is the radius of the Earth and
\(M\) is the mass of the Earth. The minimum speed with which it should be projected, so that it does not return, is:
(where
\(g\) is the acceleration due to gravity on the surface of the Earth)
| 1. |
\(\left(\dfrac{{GM}}{2 {R}}\right)^{\frac{1}{2}} \) |
2. |
\(\left(\dfrac{{g} R}{4}\right)^{\frac{1}{2}} \) |
| 3. |
\( \left(\dfrac{2 g}{R}\right)^{\frac{1}{2}} \) |
4. |
\(\left(\dfrac{G M}{R}\right)^{\frac{1}{2}}\) |
19. A current of \(2.5~\text A\) flows through a coil of inductance \(5~\text H.\) The magnetic flux linked with the coil is:
1. \(0.5~\text{Wb}\)
2. \(12.5~\text{Wb}\)
3. zero
4. \(2~\text{Wb}\)
20. The reddish appearance of the sun at sunrise and sunset is due to:
| 1. |
the scattering of light. |
| 2. |
the polarisation of light. |
| 3. |
the colour of the sun. |
| 4. |
the colour of the sky. |
21. An electron in hydrogen atom makes a transition
\(n_1 \rightarrow n_2\) where
\(n_1\) and
\(n_2\) are principal quantum numbers of the two states. Assuming Bohr's model to be valid, the time period of the electron in the initial state is eight times that in the final state. The possible values of
\(n_1\) and
\(n_2\) are:
| 1. |
\( n_1 = 6~\text{and}~n_2 = 2\) |
2. |
\( n_1 = 8~\text{and}~ n_2 = 1\) |
| 3. |
\( n_1 = 8~\text{and}~ n_2 = 2\) |
4. |
\(n_1 = 4~\text{and}~n_2 = 2\) |
22. A person holding a rifle (mass of person and rifle together is \(100\) kg) stands on a smooth surface and fires \(10\) shots horizontally, in \(5\) s. Each bullet has a mass of \(10~\mathrm{g}\) with a muzzle velocity of \(800\) ms-1. The final velocity acquired by the person and the average force exerted on the person are:
1. \(-0.08~ \text{ms}^{-1} ; 16~ \text{N} \)
2. \(-0.8~ \text{ms}^{-1} ; 16~ \text{N} \)
3. \(-1.6~ \text{ms}^{-1} ; 16~ \text{N} \)
4. \(-1.6 ~\text{ms}^{-1} ; 8 ~\text{N}\)
23. A gas in a vessel is initially at pressure
\(P.\) If the mass of all the gas molecules is halved and their speed is doubled, then the resultant pressure will be:
| 1. |
\(2 P\) |
2. |
\(P\) |
| 3. |
\(\dfrac{P}{2}\) |
4. |
\(4 P\) |
24. A circular coil
\(ABCD\) carrying a current
\(i\) is placed in a uniform magnetic field. If the magnetic force on the segment
\(AB\) is
\(\vec{ F},\) then the force on the remaining segment
\(BCDA\) is:

1.
\(-\vec{F}\)
2.
\(3\vec{F}\)
3.
\(-3\vec{F}\)
4.
\(\vec{F}\)
25. Two metal rods
\((1)\) and
\((2)\) of the same length have the same temperature difference between their ends. Their thermal conductivities are
\({K}_1\) and
\({K}_2\) and cross-sectional areas
\({A}_{1}\) and
\({A}_{2},\) respectively. If the rate of heat conduction in
\((1)\) is four times that in
\((2),\) then:
| 1. |
\(K_1 A_1=4K_2 {A}_2 \) |
2. |
\(K_1 {A}_1=2 {K}_2 {A}_2 \) |
| 3. |
\(4 {K}_1{A}_1={K}_2 {A}_2 \) |
4. |
\({K}_1 {A}_1={K}_2 {A}_2\) |
26. \(\alpha\)- particles,
\(\beta\)-particles and
\(\gamma\)-rays are all having the same energy. Their penetrating power in a given medium in increasing order will be:
| 1. |
\(\gamma , \alpha, \beta \) |
2. |
\( \alpha, \beta , \gamma \) |
| 3. |
\( \beta, \alpha , \gamma \) |
4. |
\(\beta, \gamma, \alpha \) |
27. A source of light is placed at a distance of
\(50\) cm from a photocell and the stopping potential is found to be
\(V_0\). If the distance between the light source and photocell is made
\(25\) cm, the new stopping potential will be:
| 1. |
\(V_0 /2\) |
2. |
\(V_0 \) |
| 3. |
\(4V_0 \) |
4. |
\(2V_0 \) |
28. Vectors
\(\vec {\mathrm{A}}, \vec{\mathrm{B}} \) and
\(\vec{\mathrm{C}}\) are such that
\(\vec{\mathrm{A}} \cdot \vec{\mathrm{B}}=0 \text { and } \vec{\mathrm{A}} \cdot \vec{\mathrm{C}}=0\). Then the vector parallel to
\(\vec A\) is:
| 1. |
\(\vec{A} \times \vec{B} \) |
2. |
\(\vec{B}+\vec{C} \) |
| 3. |
\(\vec{B} \times \vec{C} \) |
4. |
\(\vec{B}~\text{and} ~\vec{C}\) |
29. One way in which the operation of a
\(\text{n-p-n}\) transistor differs from that a
\(\text{p-n-p}\):
| 1. |
the emitter junction injects minority carriers into the base region of the \(\text{p-n-p}\). |
| 2. |
the emitter injects holes into the base of the \(\text{p-n-p}\) and electrons into the base regions of \(\text{n-p}\). |
| 3. |
the emitter injects holes into the base of \(\text{n-p-n}\). |
| 4. |
the emitter junction is reversed biased in \(\text{n-p}\). |
30. Which of the following relations does not give the equation of an adiabatic process, where terms have their usual meaning?
1. \({P}^{1-\gamma}{T}^{\gamma}= \text{constant}\)
2. \({PV}^{\gamma}=\text{constant}\)
3. \({TV}^{\gamma-1}= \text{constant}\)
4. \({P}^{\gamma} {T}^{1-\gamma}=\text{constant}\)
31. Two discs are rotating about their respective axes, which are normal to the discs and pass through their centres. Disc \(D_1\) has a \(2\) kg mass and \(0.2\) m radius and an initial angular velocity of \(50~\text{rad} ~\text{s}^{-1}.\) Disc \(D_2\) has a \(4\) kg mass, \(0.1\) m radius and an initial angular velocity of \(200~\text{rad} ~\text{s}^{-1}.\) The two discs are brought into contact face to face, with their axes of rotation coincident. The final angular velocity (in \(\text{rad} ~\text{s}^{-1}\)) of the combined system is:
1. \(60\)
2. \(100\)
3. \(120\)
4. \(40\)
32. The fluid is in streamlined flow through a horizontal pipe with a variable cross-sectional area. Which of the following statements is correct?
| 1. |
The velocity is maximum at the narrowest part of the pipe, and pressure is maximum at the widest part of the pipe. |
| 2. |
Both the velocity and pressure are maximum at the narrowest part of the pipe. |
| 3. |
Both the velocity and pressure are maximum at the widest part of the pipe. |
| 4. |
The velocity is minimum at the narrowest part of the pipe, and the pressure is minimum at the widest part of the pipe. |
33. An electromagnetic wave of frequency
\(\nu=3.0\) MHz passes from a vacuum into a dielectric medium with relative permittivity
\(\varepsilon =4.0.\) Then:
| 1. |
wavelength is doubled and frequency becomes half |
| 2. |
wavelength is halved and frequency remains unchanged |
| 3. |
wavelength and frequency both remain unchanged |
| 4. |
wavelength is doubled and frequency unchanged |
34. A particle with total energy
\(E\) is moving in a potential energy region
\(U(x).\) The motion of the particle is restricted to the region where:
| 1. |
\(U(x)<E\) |
2. |
\(U(x)=0\) |
| 3. |
\(U(x)\leq E\) |
4. |
\(U(x)> E\) |
35. The displacement
\(x\) (in
\(\text m\)) of a particle of mass
\(m\) (in
\(\text{kg}\)) moving in one dimension under the action of a force, is related to time
\(t\) (in
\(\text s\)) by;
\(t = (\sqrt x +3 ).\) The displacement of the particle when its velocity is zero will be:
| 1. |
\(4~\text m\) |
2. |
zero |
| 3. |
\(6~\text m\) |
4. |
\(2~\text m\) |
36. A
\(12\) cm wire is given a shape of a right-angled triangle
\(\mathrm{ABC}\) having sides
\(3\) cm,
\(4\) cm and
\(5\) cm, as shown in the figure. The resistance between two ends
\((\mathrm{AB, BC, CA})\) of the respective sides are measured one by one by a multi-meter. The resistances will be in the ratio:

1.
\(9:16:25\)
2.
\(27:32:35\)
3.
\(21:24:25\)
4.
\(3:4:5\)
37. Two sources \(\text{P}\) and \(\text{Q}\) produce notes of frequency \(660~\text{Hz}\) each. A listener moves from \(\text{P}\) to \(\text{Q}\) with a speed of \(1\) m/s. If The speed of sound is \(330\) m/s, then number of beats heard by the listener per second will be:
1. \(4\)
2. \(8\)
3. \(2\)
4. zero
38. The de-Broglie wavelength of neutrons in thermal equilibrium at temperature \(T\) is:
1. \(\dfrac{3.08}{\sqrt{T}} ~\mathring{A}\)
2. \(\dfrac{0.308}{\sqrt{T}} ~\mathring{A}\)
3. \(\dfrac{0.0308}{\sqrt{T}} ~\mathring{A}\)
4. \(\dfrac{30.8}{\sqrt{T}} ~\mathring{A}\)
39. Two Carnot engines A and B are operated in series. Engine
\(A\) receives heat from the source at temperature
\(T_1\) and rejects the heat to the sink at temperature
\(T.\) The second engine
\(B\) receives the heat at temperature
\(T\) and rejects to its sink at temperature
\(T_2\). For what value of
\(T\) the efficiencies of the two engines are equal?
| 1. |
\(\dfrac{T_1-T_2}{2} \) |
2. |
\(T_1 T_2 \) |
| 3. |
\(\sqrt{T_1 T_2} \) |
4. |
\(\dfrac{T_1+T_2}{2}\) |
40. A particle of mass
\(m\) oscillates along the
\({x}\text-\)axis according to the equation
\(x = a {\sin} \omega t.\) The nature of the graph between momentum and displacement of the particle is:
| 1. |
circle |
| 2. |
hyperbola |
| 3. |
ellipse |
| 4. |
a straight line passing through the origin |
41. Ten identical cells connected in series are required to heat a wire of length
\(1\) metre and radius
\(r\) by
\(10^\circ ~\text{C}\) in time
\(t.\) How many cells will be needed to heat a wire of length
\(2\) metres with the same radius by the same temperature in the same time
\(t?\)
| 1. |
\(20\) |
2. |
\(30\) |
| 3. |
\(40\) |
4. |
\(10\) |
42. The density of water at
\(20^\circ \text{C}\) is
\(998~\text{kg/m}^3\) and at
\(40^\circ \text{C}\) is
\(992~\text{kg/m}^3.\) The coefficient of volume expansion of water is:
| 1. |
\(3 \times 10^{-4} / ^\circ\text C\) |
2. |
\(2 \times 10^{-4} / ^\circ\text C\) |
| 3. |
\(6 \times 10^{-4} / ^\circ\text C\) |
4. |
\(10^{-4} / ^\circ\text C\) |
43. Two plane mirrors are inclined at
\(70^\circ.\) A ray incident on one mirror at an angle
\(\theta\) after reflection falls on the second mirror and is reflected from there parallel to the first mirror. The value of
\(\theta\) is:
| 1. |
\(45^\circ\) |
2. |
\(30^\circ\) |
| 3. |
\(55^\circ\) |
4. |
\(50^\circ\) |
44. In an unbiased
\(\mathrm{p\text{-}n}\) junction, holes diffuse from the
\(\mathrm{p\text{-}}\)regions to
\(\mathrm{n\text{-}}\)regions because of:
| 1. |
the attraction of free electrons of the \(\mathrm{n\text{-}}\)region. |
| 2. |
the higher hole concentration in the \(\mathrm{p\text{-}}\)region than that in the \(\mathrm{n\text{-}}\)region. |
| 3. |
the higher concentration of electrons is in the \(\mathrm{p\text{-}}\)region than that in the \(\mathrm{n\text{-}}\)region. |
| 4. |
the potential difference across the \(\mathrm{p\text{-}n}\) junction. |
45. One coolie takes \(1\) minute to raise a suitcase through a height of \(2~\text m\) but the second coolie takes \(30~\text s\) to raise the same suitcase to the same height. The powers of two coolies are in the ratio:
1. \(1:3\)
2. \(2:1\)
3. \(3:1\)
4. \(1:2\)
Chemistry
46. The outer electronic configuration of Gd:
( At. No. 64)
1. 4f
5 5d
4 6s
1
2. 4f
7 5d
1 6s
2
3. 4f
3 5d
5 6s
2
4. 4f
4 5d
5 6s
1
47. Accumulation of lactic acid (HC3H5O3 a monobasic acid), in tissues leads to pain and fatigue. In a 0.10 M aqueous solution, lactic acid is 3.7% dissociated. The value of the dissociation constant, Ka, for this acid, will be:
1. 1.4 × 10–5
2. 1.4 × 10–4
3. 3.7 × 10–4
4. 2.8 × 10–4
48. For a reaction between A and B the order with respect to A is 2 and the order with respect to B is 3. The concentration of both A and B is doubled the rate will increase by a factor of:
1. 12
2. 16
3. 32
4. 10
49. The metal oxide which cannot be reduced to metal by carbon is:
1. Al2O3
2. PbO
3. ZnO
4. Fe2O3
50. Given:
 |
 |
| I |
II |
I and II are classified as:
1. Identical
2. A pair of conformers
3. A pair of geometrical isomers
4. A pair of optical isomers
51. Crystal field splitting energy for high spin d
4 octahedral complex is:
| 1. |
– 1.2 \(\Delta_{0}\) |
2. |
– 0.6 \(\Delta_{0}\) |
| 3. |
– 0.8 \(\Delta_{0}\) |
4. |
–1.6 \(\Delta_{0}\) |
52. Arrange the following in increasing order of stability:
| (a) |
\(\left(\mathrm{CH}_3\right)_2 \stackrel{\oplus}{\mathrm{C}}-\mathrm{CH}_2-\mathrm{CH}_3 \) |
(b) |
\(\left(\mathrm{CH}_3\right)_3 \stackrel{\oplus}{\mathrm{C}} \) |
| (c) |
\(\left(\mathrm{CH}_3\right)_2 \stackrel{\oplus}{\mathrm{C}}\mathrm{H} \) |
(d) |
\( \text {CH}_3 \stackrel{\oplus}{\mathrm{C}}\mathrm{H}_2\) |
| (e) |
\( \stackrel{\oplus}{\mathrm{C}}\mathrm{H}_3 \) |
| 1. |
e < d < c < a < b |
2. |
d < e < c < a < b |
| 3. |
a < e < d < c < b |
4. |
e < d < c < b < a |
53. Consider the half-cell reduction reaction:
\(\text{Mn}^{2+}+2e^-\rightarrow \text{Mn},\ E^{0}= -1.18~ \text V \)
\(\text{Mn}^{2+}\rightarrow \text{Mn}^{3+}+e^-,\ E^{0}= -1.51~ \text V \)
The
\(E^{0}\) for the reaction
\(\mathrm{3\ Mn^{2+}\rightarrow Mn^{0}+2Mn^{3+} }\) and possibility of the forward reaction are respectively:
| 1. |
–4.18 V and Yes |
2. |
+0.33 V and Yes |
| 3. |
+2.69 V and No |
4. |
–2.69 V and No |
54. How many grams of cobalt metal will be deposited when a solution of cobalt (II) chloride is electrolyzed with a current of 10 amperes for 109 minutes?
(Given: 1 Faraday = 96,500 C; Atomic mass of Co = 59 u)
| 1. |
4.0 |
2. |
20.0 |
| 3. |
40.0 |
4. |
0.66 |
55. In a particular isomer of [Co(NH
3)
4Cl
2]°, the
Cl–Co–Cl angle is 90°, and the isomer is known as:
| 1. |
Optical isomer |
2. |
Cis-isomer |
| 3. |
Position isomer |
4. |
Linkage isomer |
56. Which one of the following statements is not true?
| 1. |
Clean water would have a BOD value of 5 ppm. |
| 2. |
Fluoride deficiency in drinking water is harmful. Soluble fluoride is often used to bring its concentration upto 1 ppm. |
| 3. |
When the pH of rainwater is higher than 6.5, it is called acid rain. |
| 4. |
Dissolved Oxygen (DO) in cold water can reach a concentration upto 10 ppm. |
57. When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litres of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (
\(\Delta \)H
comb. (CH
4) = 890 kJ mol
–1,
\(\Delta \)H
comb( C
3H
8) = 2220 kJ mol
–1) is:
| 1. |
38 |
2. |
317 |
| 3. |
477 |
4. |
32 |
58. The hybridisation of benzyl carbonium ion

is:
| 1. |
sp2 |
2. |
spd2 |
| 3. |
sp2d |
4. |
sp3 |
59. The pair of species that has the same bond order in the following is:
| 1. |
CO, NO+ |
2. |
NO–, CN– |
| 3. |
O2, N2 |
4. |
O2, B2 |
60. According to the law of photochemical equivalence, the energy absorbed (in ergs/mole) is given as:
(h = 6.62 × 10
–27 ergs, c = 3 × 10
10 cm s
–1, N
A = 6.02 × 10
23 mol
–1)
| 1. |
\({{1.196\times 10^{8}}\over{\lambda}}\) |
2. |
\({{2.859\times 10^{5}}\over{\lambda}}\) |
| 3. |
\({{2.859\times 10^{16}}\over{\lambda}}\) |
4. |
\({{1.196\times 10^{16}}\over{\lambda}}\) |
61. Three thermochemical equations are given below:
| (i) |
C(graphite) + O2(g) \(\rightarrow\) CO2(g); \(\Delta_{r}\)H° = x kJ mol–1 |
| (ii) |
Cgraphite + 1/2 O2(g) \(\rightarrow\) CO(g); \(\Delta_{r}\)H° = y kJ mol–1 |
| (iii) |
CO(g) + 1/2 O2(g) \(\rightarrow\) CO2(g); \(\Delta_{r}\)H° = z kJ mol–1 |
Based on the above equations, find out which one of the relationships given below is correct :
| 1. |
z = x + y |
2. |
x = y + z |
| 3. |
y = 2z – x |
4. |
x = y – z |
62. What is the correct order of electron gain enthalpy from least negative to most negative for the elements C, Ca, Al, F, and O?
1. Al < Ca < O < C < F
2. Al < O < C < Ca < F
3. C < F < O < Al < Ca
4. Ca < Al < C < O < F
63. The anion of acetylacetone (acac) forms Co(acac)3 chelate with Co3+. The rings of the chelate are:
1. Five membered
2. Four membered
3. Six membered
4. Three membered
64. At 100 °C the K
w of water is 55 times its value at 25ºC. What will be the pH of a neutral solution?
(log 55 = 1.74)
| 1. |
7.00 |
2. |
7.87 |
| 3. |
5.13 |
4. |
6.13 |
65. Identify the incorrect statements, regarding the molecule XeO4:
1. XeO4 molecule is square planar
2. There are four p\(\pi\) – d\(\pi\) bonds
3. There are four sp3 – p, \(\sigma\) bonds
4. XeO4 molecule is tetrahedral
66. In Castner-Kellner cell for the production of sodium hydroxide:
1. brine is electrolyzed using graphite electrodes
2. molten sodium chloride is electrolysed
3. sodium amalgam is formed at mercury cathode
4. brine is electrolyzed with Pt electrodes
67. Which of the following chemical system is non-aromatic?
68. Phenol is distilled with Zn dust followed by Fridel Crafts alkylation with propyl chloride in the presence of AlCl
3 to give a compound (B). (B) is oxidized in the presence of air to form the compound (C). The structural formula of (C) is:
69. Nitrogen detection in an organic compound is carried out by Lassaigne's test. The blue colour formed corresponds to which of the following formulae:
1. \(\mathrm{Fe_3[Fe(CN)_6]_2}\)
2. \(\mathrm{Fe_4[Fe(CN)_6]_3}\)
3. \(\mathrm{Fe_4[Fe(CN)_6]_2}\)
4. \(\mathrm{Fe_3[Fe(CN)_6]_3}\)
70. \(\mathrm{CH_3-CH_3, \ CH_3-CH_2-CH_3, \ }\)
\(\mathrm{\left(CH_3\right)_2 CH-CH_3, }\) and \(\mathrm{CH_3-CH_2-CH\left(CH_3\right)_2 }\)
The increasing order of stability of the free radicals formed from homolytic fission of the above three alkanes is:
| 1. |
\(\mathrm{ (CH_3)_2 \dot{C}-CH_2 CH_3<CH_3-\dot{C} H-CH_3 \\ <CH_3-\dot{C} H_2<(CH_3)_3 \dot{C} }\) |
| 2. |
\( \mathrm{CH_3-\dot{C} H_2<CH_3-\dot{C} H-CH_3 \\ <\left(CH_3\right)_2 \dot{C}-CH_2 CH_3<\left(CH_3\right)_3 \dot{C}}\) |
| 3. |
\(\mathrm{CH_3-\dot{C} H_2<CH_3-\dot{C} H-CH_3\\ <\left(CH_3\right)_3 \dot{C}<\left(CH_3\right)_2 \dot{C}-CH_2 CH_3 }\) |
| 4. |
\(\mathrm{ \left(CH_3\right)_3 \dot{C}<\left(CH_3\right)_2 \dot{C}-CH_2-CH_3 \\ <CH_3-\dot{C} H-CH_3<CH_3-\dot{C} H_2 }\) |
71. Incorrect statement among the following is:
| 1. |
Quartz is extensively used as a piezoelectric material. |
| 2. |
Silicones are a group of organosilicon polymers, which have (-R2SiO-) as a repeating unit. |
| 3. |
Basic structural unit in silicates is the SiO44– tetrahedron. |
| 4. |
Feldspars are not aluminosilicates. |
72. The outer orbitals of C in an ethene molecule can be considered to be hybridized to given three equivalent sp2 orbitals. The total number of sigma (\(\sigma\)) and pi(\(\pi\)) bonds in ethene molecule are:
1. 3 sigma (\(\sigma\)) and 2 pi(\(\pi\)) bonds
2. 4 sigma (\(\sigma\)) and 1 pi(\(\pi\)) bonds
3. 5 sigma (\(\sigma\)) and 1 pi(\(\pi\)) bonds
4. 1 sigma (\(\sigma\)) and 2 pi(\(\pi\)) bonds
73. Which condition is not satisfied by an ideal solution?
| 1. |
\(\Delta_{mix} V\) = 0 |
2. |
\(\Delta_{mix} S\) = 0 |
| 3. |
Obeyance to Roult's Law |
4. |
\(\Delta_{mix} H\) = 0 |
74. The dissociation constant of a weak acid is
\(1\times 10^{-4}\) in order to prepare a buffer solution with pH=5 the [salt]/ [Acid] ratio should be:
| 1. |
4 : 5 |
2. |
10 : 1 |
| 3. |
5 : 4 |
4. |
1 : 10 |
75. What is the density of N2 gas at 227 °C and 5.00 atm pressure?
(R = 0.082 L Atm K–1 mol–1)
1. 1.40 g/L
2. 2.81 g/L
3. 3.41 g/L
4. 0.29 g/L
76. The correct IUPAC name for [CrF
2(en)
2]Cl is:
| 1. |
Chlorodifluoridoethylenediaminechromium(III) chloride |
| 2. |
Bis-(ethylenediamine)difluoridochromium(III) chloride |
| 3. |
Difluorobis-(ethylenediamine)chromium(III) chloride |
| 4. |
Chlorodifluoridobis(ethylenediamine)chromium (III) |
77. Dettol is the mixture of:
1. Chloroxylenol and Bithionol
2. Chloroxylenol and Terpineol
3. Phenol and Iodine
4. Terpineol and Bithionol
78. A reaction is 50% complete in 2 hours and 75% complete in 4 hours. The order of the reaction is:
79. The values of \(K_{sp}\) of CaCO3 and CaC2O4 are 4.7 × 10–9 and 1.3 × 10–9 respectively at 25°C. If the mixture of these two is washed with water, what is the concentration of Ca2+ ions in the water?
1. 5.8 × 10–5 M
2. 6.8 × 10–5 M
3. 3.6 × 10–5 M
4. 7.7 × 10–5 M
80. The species/pair among the following that have sp3 hybridization is:
1. SiF4, BeH2
2. NF3,H2O
3. NF3,BF3
4. H2S, BF3
81. In DNA, the linkages between different nitrogenous bases are:
1. Phosphate linkages
2. H-bonding
3. Glycosidic linkages
4. Peptide linkages
82. Which complex among the following exhibits a paramagnetic nature?
1. [Co(NH3)6]3+
2. [Pt(en)Cl2]
3. [CoBr4]2–
4. Mo(CO)6
(At. No. Mo = 42, Pt = 78)
83. Sc(Z = 21) is a transition element but Zn (Z = 30) is not because:
| 1. |
Both Sc3+ and Zn2+ ions are colourless and form white compounds |
| 2. |
In the case of Sc, 3d orbitals are partially filled but in Zn, these are fully filled. |
| 3. |
The last electron is assumed to be added to the 4s level in the case of Zn |
| 4. |
Both Sc and Zn do not exhibit variable oxidation states. |
84. In an experiment, it showed that 10 mL of 0.05 M solution of chloride required 10 mL of 0.1 M solution of AgNO3. Which of the following will be the formula of the chloride (X stands for the symbol of the element other than chlorine)?
1. X2Cl2
2. XCl2
3. XCl4
4. X2Cl
85. A diamagnetic compound among the following is:
1. [Co(F6)]3–
2. [Ni(CN)4]2–
3. [NiCl4]2–
4. [Fe(CN)6]3–
86. A compound undergoes hydrolysis, resulting in the formation of two products. One of these products, when treated with sodium nitrite and hydrochloric acid, yields a product that does not respond to the iodoform test. The other product is capable of reducing Tollen's reagent and Fehling's solution.
Based on these observations, the compound is:
1. CH3CH2CH2NC
2. CH3CH2CH2CN
3. CH3CH2CH2ON = O
4. CH3CH2CH2CON(CH3)2
87. Some reactions of amines are given. Which one is not correct?
| 1. |
 |
| 2. |
\(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2+\mathrm{HNO}_2 \rightarrow \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}+\mathrm{N}_2 \) |
| 3. |
\(\mathrm{CH}_3 \mathrm{NH}_2+\mathrm{C}_6 \mathrm{H}_5 \mathrm{SO}_2 \mathrm{Cl} \rightarrow \mathrm{CH}_3 \mathrm{NHSO}_2 \mathrm{C}_6 \mathrm{H}_5 \) |
| 4. |
\(\left(\mathrm{CH}_3\right)_2 \mathrm{NH}+\mathrm{NaNO}_2+\mathrm{HCl} \rightarrow\left(\mathrm{CH}_3\right)_2 \mathrm{~N}-\mathrm{N}=\mathrm{O} \) |
88. In which of the following ionization processes the bond energy increases and the magnetic behaviour changes from paramagnetic to diamagnetic?
1. \(\mathrm{O_{2}\rightarrow O_{2}^{+} }\)
2. \(\mathrm{C_{2}\rightarrow C_{2}^{+} }\)
3. \(\mathrm{NO\rightarrow NO^{+} }\)
4. \(\mathrm{N_{2}\rightarrow N_{2}^{+} }\)
89. In the following reaction:
\(\text {HC} \equiv \text {CH} \xrightarrow [Hg^{2+}] { H_2SO_4} P \)
Product 'P' will not give
1. Tollen's reagent test
2. Fehling's solution
3. Victor Meyer test
4. Iodoform test
90. The number of isomeric alcohols (excluding stereoisomers) of molecular formula C6H14O which give a positive iodoform test are:
1. Three
2. Four
3. Five
4. Two
Biology
91. Figure shows blood circulation in humans with labels A to D. Select the option which gives correct identification of label and functions of the part:

| 1. |
B - Capillary - thin without muscle layers and wall two-cell layer thick |
| 2. |
C - Vein - thin-walled and blood flows in jerks/spurts |
| 3. |
D - Pulmonary vein - takes oxygenated blood to heart PO2=95 mm Hg |
| 4. |
A - Artery- thick-walled and blood flows evenly |
92. Which enzymes are likely to act on the baked potatoes eaten by a man, starting from the mouth and as it moves down the alimentary canal?
| 1. |
Pancreatic amylase → salivary amylase → lipases |
| 2. |
Disaccharidase like maltase → lipases → nucleases |
| 3. |
Salivary amylase → pancreatic amylase → disaccharidases |
| 4. |
Salivary maltase → carboxy peptidase → trypsinogen |
93. The largest tiger reserve in India is:
| 1. |
Valmiki |
2. |
Nagarjunsagar - Srisailam |
| 3. |
Periyar |
4. |
Nagarhole |
94. The characteristics of class Reptilia are:
| 1. |
Body covered with moist skin which is devoid of scales, the ear is represented by a tympanum, alimentary canal, urinary and reproductive tracts open into a common cloaca |
| 2. |
Freshwater animals with a bony endoskeleton and air bladder to regulate buoyancy |
| 3. |
Marine animals with cartilaginous endoskeletons, bodies covered with placoid scales |
| 4. |
Body covered with dry and cornified skin, scales over the body are epidermal, they do not have external ears |
95. Among flowers of
Calotropis, tulip,
Sesbania,
Asparagus, Colchicine, Sweet pea,
Petunia, Indigofera, Mustard, Soybean, Tobacco and groundnut how many plants have corolla with valvate aestivation?
| 1. |
Six |
2. |
Seven |
| 3. |
Eight |
4. |
Five |
96. During muscle contraction in humans the:
| 1. |
Sarcomere does not shorten |
2. |
A band remains the same |
| 3. |
A, H and I bands shorten |
4. |
Actin filaments shorten |
97. Which one of the following statements is correct regarding Sexually Transmitted Diseases (STD)?
| 1. |
A person may contact syphilis by sharing milk with one already suffering from the disease |
| 2. |
Haemophilia is one of the STD |
| 3. |
Genital herpes and sickle-cell, anemia are both STD |
| 4. |
The chances of a 5-year boy contacting a STD are very little |
98. During meiosis I, the chromosomes start pairing at:
| 1. |
Zygotene |
2. |
Pachytene |
| 3. |
Diplotene |
4. |
Leptotene |
99. Identify the tissue shown in the diagram and match with its characteristics and its location:

| 1. |
Smooth muscles, show branching, found in the walls of the heart |
| 2. |
Cardiac muscles, unbranched muscles, found in the walls of the heart |
| 3. |
Striated muscles, tapering at both ends attached to the bones of the ribs |
| 4. |
Skeletal muscle, shows striations and is closely attached to the bones of the limbs |
100. One of the most frequently used techniques in DNA fingerprinting is:
1. VNTR
2. SSCP
3. SCAR
4. AFLP
101. The common characteristics between tomato and potato will be maximum at the level of their:
| 1. |
Family |
2. |
Order |
| 3. |
Division |
4. |
Genus |
102. The figure shows a human blood cell. Identify it and give its characteristics:

|
Blood cell |
Characteristics |
| 1. |
Basophils |
Secrete serotonin |
| 2. |
B-lymphocytes |
form about 20% of blood cells involved in immune response |
| 3. |
Neutrophils |
Most abundant blood cells, phagocytic |
| 4. |
Monocytes |
Life spans 3 days, produce antibodies |
103. Random unidirectional change in allele frequencies that occur by chance in all populations and especially in small populations is known as:
| 1. |
Migration |
2. |
Natural selection |
| 3. |
Genetic drift |
4. |
Mutation |
104. The plant body is thalloid in:
| 1. |
Sphagnum |
2. |
Salvinia |
| 3. |
Marchantia |
4. |
Funaria |
105. Select the correct option with respect to Cockroaches:
| 1. |
Malpighian tubules convert nitrogenous wastes into urea |
| 2. |
Males bear short and styles not present in females |
| 3. |
Nervous system comprises a dorsal nerve cord and ten pairs of ganglion |
| 4. |
The fore wings are tegmina which are used in flight |
106. During the metaphase stage of mitosis spindle fibres attach to chromosomes at:
| 1. |
kinetochore |
| 2. |
both centromere and kinetochore |
| 3. |
centromere, kinetochore and areas adjoining centromere |
| 4. |
centromere |
107. Select the alternative giving correct identification and function of the organelle 'A' in the diagram:

| 1. |
Mitochondria - produce cellular energy in the form of ATP |
| 2. |
Golgi body - provides packaging material |
| 3. |
Lysosomes - secrete hydrolytic enzymes |
| 4. |
Endoplasmic reticulum - synthesis of lipids |
108. RNA interference involves:
| 1. |
Synthesis of cDNA from RNA using reverse transcriptase |
| 2. |
Silencing of specific mRNA due to complementary RNA |
| 3. |
Interference of RNA in the synthesis of DNA |
| 4. |
Synthesis of mRNA from DNA |
109. Which one of the following groups of animals reproduces only by sexual means?
| 1. |
Cnidaria |
2. |
Porifera |
| 3. |
Protozoa |
4. |
Ctenophora |
110. The figure shows a section of the human ovary. Select the option which gives the correct identification of A and B with function/characteristic:

| 1. |
B - Corpus luteum - secretes progesterone |
| 2. |
A - Tertiary follicle - forms Graafian follicle |
| 3. |
B - Corpus luteum - secretes LH |
| 4. |
A - Primary oocyte - it is the prophase - I of the meiotic division |
111. The stage transferred into the uterus after induced fertilization of ova in the laboratory is:
1. embryo at 4 blastomere stage
2. embryo at 2 blastomere stage
3. Morula
4. Zygote
112. The figure shows a hypothetical tetrapeptide portion of a protein with parts labelled A-D. Which one of the following options is correct?

| 1. |
D is the acidic amino acid - glutamic acid |
| 2. |
C is an aromatic amino acid - tryptophan |
| 3. |
A is the C-terminal amino acid and D is N terminal amino acid |
| 4. |
A is the sulphur containing amino acid-methionine |
113. Identify the site where Wuchereria bancrofti is normally found on human body.
1. Muscles of the legs
2. Blood vessels of the thigh region
3. Skin between the fingers
4. Lymphatic vessels of the lower limbs
114. In our society women are blamed for producing female children. Choose the correct answer for sex-determination in humans.
| 1. |
due to some defect like aspermia in man |
| 2. |
due to the genetic makeup of the particular sperm which fertilizes the egg |
| 3. |
due to the genetic makeup of the egg |
| 4. |
due to some defect in the women |
115. Genetic variation in a population arises due to:
1. Recombination only
2. Mutations as well as recombination
3. Reproductive isolation and selection
4. Mutations only
116. The second commitment period for Kyoto Protocol was decided at:
1. Durban
2. Bali
3. Doha
4. Cancun
117. Climate of the world is threatened by:
1. Decreasing amount of atmospheric oxygen
2. Increasing amount of atmospheric carbon dioxide
3. Decreasing amount of atmospheric carbon dioxide
4. Increasing concentration of atmospheric oxygen
118. Which of the following statement is not true for stomatal apparatus?
| 1. |
Guard cells invariably possess chloroplasts and mitochondria |
| 2. |
Guard cells are always surrounded by subsidiary cells |
| 3. |
Stomata are involved in gaseous exchange |
| 4. |
Inner walls of guard cells are thick |
119. The age pyramid with broad base indicates:
1. high percentage of old individuals
2. low percentage of young individuals
3. a stable population
4. high percentage of young individuals
120. Select the option which shows the correct matching of animal with excretory organs and excretory product:
|
Animal |
Excretory organs |
Excretory product |
| 1. |
Labeo (Rohu) |
Nephridial tubes |
Ammonia |
| 2. |
Salamander |
Kidney |
Urea |
| 3. |
Peacock |
Kidney |
Urea |
| 4. |
Housefly |
Renal tubules |
Uric acid |
121. Which of the following best illustrates FEEDBACK in development?
| 1. |
Tissue (X) secretes RNA which changes the development of tissue (Y) |
| 2. |
As tissue (X) develops, it secretes enzymes that inhibit the development of tissue (Y) |
| 3. |
As tissue (X) develops, it secretes something that induces tissue (Y) to develop |
| 4. |
As tissues (X) develops, it secretes something that shows down the growth of tissue (Y) |
122. Which of the following type of plastids does not contain stored food material?
1. Chromoplasts
2. Eleioplasts
3. Aleuroplasts
4. Amyloplasts
123. Tissue culture technique can produce an infinite number of new plants from a small parental tissue. The economic importance of the technique is in raising:
| 1. |
genetically uniform population identical to the original parent |
| 2. |
homozygous diploid plants |
| 3. |
development of new species |
| 4. |
variants through picking up somaclonal variations |
124. Inflorescence is racemose in:
1. Brinjal
2. Tulip
3. Aloe
4. Soybean
125. Which one of the following is wrongly matched?
1. Spirogyra -Motile gametes
2. Sargassum - Chlorophyll C
3. Basidiomycetes - Puffballs
4. Nostoc- Water blooms
126. The figure shows an axon terminal and synapse. Select the option giving correct identifications of labels A-D.

1. A-Action potential C-Neurotransmitter
2. B-Neurotransmitter D-Receptor capsules
3. C-Receptor D-Synaptic vesicles
4. A-Axon terminal B-Serotonin complex
127. Which one of the following vectors is used to replace the defective gene in gene therapy?
1. Adenovirus
2. Cosmid
3. Ri plasmid
4. Ti plasmid
128. Animal vectors are required for pollination in:
| 1. |
Vallisneria |
2. |
Mulberry |
| 3. |
Cucumber |
4. |
Maize |
129. Which one of the following is not a parasitic adaptation?
1. Development of adhesive organs
2. Loss of digestive organs
3. Loss of reproductive capacity
4. Loss of unnecessary sense organs
130. The pineapple which under natural conditions is difficult to blossom has been made to produce fruits throughout the year by application of:
1. NAA, 2, 4-D
2. GA3
3. Cytokinin
4. IAA, IBA
131. A sagittal section of the human brain is shown here. Identify at least two labels from A-D:

1. C-Mid brain; D-Cerebellum
2. A-Cerebrum; C-Pons
3. B-Corpus callosum; D-Medulla
4. A-Cerebral hemispheres; B-Cerebellum
132. Microbe used for biocontrol of pest butterfly caterpillars is:
1. Saccharomyces cerevisiae
2. Bacillus thuringiensis
3. Streptococcus sp.
4. Trichoderma sp.
133. Down's syndrome in humans is due to:
1. Three 'X' chromosomes
2. Three copies of chromosome 21
3. Monosomy
4. Two 'Y' chromosomes
134. The viability of seeds is tested by:
1. 2, 6 dichlorophenol indophenols
2. 2, 3, 5 triphenyl tetrazolium chloride
3. DMSO
4. Safranine
135. What is common in all the three,
Funaria,
Dryopteris and Ginkgo?
| 1. |
Presence of archegonia |
| 2. |
Well-developed vascular tissues |
| 3. |
Independent gametophyte |
| 4. |
Independent sporophyte |
136. Albuminous seeds store their reserve food mainly in:
1. Endosperm
2. Cotyledons
3. Hypocotyl
4. Perisperm
137. Megaspores are produced from the megaspore mother cells after:
1. Mitotic division
2. Formation of a thick wall
3. Differentiation
4. Meiotic division
138. Benthic organisms are affected most by:
1. Light reaching the forest floor
2. Surface turbulence of water
3. Sediment characteristics of aquatic ecosystems
4. Water-holding capacity of soil
139. Bundle sheath cells:
| 1. |
are rich in PEP carboxylase |
| 2. |
lack RuBisCo |
| 3. |
lack both RuBisCo and PEP carboxylase |
| 4. |
are rich in RuBisCo |
140. Meristematic tissue responsible for the increase in girth of a tree trunk is:
1. Intercalary meristem
2. Lateral meristem
3. Phellogen
4. Apical meristem
141. Which one of the following animals is correctly matched with its one characteristic and the taxon?
|
Animal |
Characteristic |
Taxon |
| 1. |
Millipede |
Ventral nerve cord |
Arachnids |
| 2. |
Sea Anemone |
Triploblastic |
Cnidaria |
| 3. |
Silverfish |
Pectoral and pelvic fins |
Chordata |
| 4. |
Duckbilled platypus |
Oviparous |
Mammalian |
142. Which one of the following is not correct as regards the harmful effects of particulate matter of the size 2.5 micrometers or less?
1. It can cause respiratory problems
2. It can directly enter into our circulatory system
3. It can cause inflammation and damage to the lungs
4. It can be inhaled into the lungs
143. Which of the following has maximum genetic diversity in India?
| 1. |
Mango |
2. |
Wheat |
| 3. |
Groundnut |
4. |
Rice |
144. Which one is the incorrect statement with regards to the importance of pedigree analysis?
| 1. |
It confirms that DNA is the carrier of genetic information |
| 2. |
It helps to understand whether the trait in question is dominant or recessive |
| 3. |
It confirms that the trait is linked to one of the autosomes |
| 4. |
It helps to trace the inheritance of a specific trait |
145. Specialized cells for fixing atmospheric nitrogen in
Nostoc are:
| 1. |
Heterocysts |
2. |
Hormogonia |
| 3. |
Nodules |
4. |
Akinetes |
146. One of the following is not a method of contraception- which one?
1. condoms
2. pills of a combination of oxytocin and vasopressin
3. Lippes loop
4. tubectomy
147. A stage of mitosis is shown in the diagram. Which stage is it and what are its characteristics?

| 1. |
Metaphase-spindle fibers attached to kinetochores, centromeres split and chromatids separate |
| 2. |
Metaphase- chromosomes moved to spindle equator, chromosomes made up of two sister chromatids |
| 3. |
Anaphase - centromeres split and chromatids separate and start moving away |
| 4. |
Late prophase - chromosomes move to spindle equator |
148. In a cymose inflorescence, the main axis:
| 1. |
has unlimited growth |
| 2. |
bears a solitary flower |
| 3. |
has unlimited growth but lateral branches end in flowers |
| 4. |
terminates in a flower |
149. Sharks and dogfishes differ from skates and rays by:
| 1. |
Gill slits are ventrally placed |
| 2. |
Head and trunk are widened considerably |
| 3. |
Distinct demarcation between body and tail |
| 4. |
Their pectorals fins are distinctly marked off from cylindrical bodies |
150. The term 'glycocalyx' is used for:
| 1. |
a layer present between cell wall and membrane of bacteria |
| 2. |
cell wall of bacteria |
| 3. |
bacterial cell glyco-engineered to possess N-glycosylated proteins |
| 4. |
a layer surrounding the cell wall of bacteria |
151. In an inducible operon, the genes are:
1. usually not expressed unless a signal turns them "on"
2. usually expressed unless a signal turns them "off"
3. never expressed
4. always expressed
152. Select the option which correctly matches the endocrine gland with its hormone and its function:
|
Endocrine gland |
Hormone |
Functions |
| 1. |
Placenta |
estrogen |
initiates secretion of milk |
| 2. |
Corpus luteum |
estrogen |
essential for the maintenance of endometrium |
| 3. |
Leydig cells |
androgen |
initiates the production of sperm |
| 4. |
Ovary |
FSH |
stimulates follicular development and the secretion of estrogens |
153. Genes of interest can be selected from a genomic library by using:
1. Cloning vectors
2. DNA probes
3. Gene targets
4. Restriction enzymes
154. A healthy person eats the following diet-5gm raw sugar, 4 gm albumin, 10 gm pure buffalo ghee adulterated with 2 gm vegetable ghee (hydrogenated vegetable oil) and 5 gm lignin. How many calories he is likely to get?
1. 126
2. 164
3. 112
4. 144
155. Select the correct statement with respect to disorders of muscles in humans.
| 1. |
Failure of neuromuscular transmission in myasthenia gravis can prevent normal swallowing |
| 2. |
Accumulation of urea and creatine in the joints causes their inflammation |
| 3. |
An overdose of vitamin D causes osteoporosis |
| 4. |
Rapid contractions of skeletal muscles causes muscle dystrophy |
156. How many plants among China rose, Ocimum, sunflower, mustard, Alstonia, guava, Calotropis and Nerium (Oleander) have opposite phyllotaxy?
1. Three
2. Four
3. Five
4. Two
157. Which of the following statements is not true about somatic embryogenesis?
| 1. |
The pattern of development of a somatic embryo is comparable to that of a zygotic embryo |
| 2. |
Somatic embryos can develop from microspores |
| 3. |
Somatic embryo is induced usually by an auxin such as 2, 4–D |
| 4. |
A somatic embryo develops from a somatic cell |
158. Which one of the following statements is correct?
| 1. |
Cleistogamous flowers are always autogamous |
| 2. |
Xenogamy occurs only by wind pollination |
| 3. |
Chasmogamous flowers do not open at all |
| 4. |
Geitonogamy involves the pollen and stigma of flowers of different plants |
159. Norepinephrine:
a. is released by sympathetic fibers
b. is released by parasympathetic fibers
c. increases the heart rate
d. decreases blood pressure
Which of the above said statements are correct?
| 1. |
a and c |
2. |
b and c |
| 3. |
b and d |
4. |
a and d |
160. Dinosaurs dominated the World in which of the following geological era?
1. Coenozoic
2. Jurassic
3. Mesozoic
4. Devonion
161. Which of the following statements is correct?
| 1. |
Sporopollenin can be degraded by enzymes |
| 2. |
Sporopollenin in made up of inorganic materials |
| 3. |
Sporopollenin can withstand high temperatures as well as strong acids and alkalis |
| 4. |
Sporopollenin can withstand high temperatures but not strong acids |
162. Which one of the following is a hallucinogenic drug?
1. Caffeine
2. Morphine
3. Lysergic acid diethylamide
4. Opium
163. The finch species of Galapagos Islands are grouped according to their food sources. Which of the following is not a finch food?
| 1. |
Carrion |
2. |
Insects |
| 3. |
Tree buds |
4. |
Seeds |
164. Which of the following is not a property of the genetic code?
1. Non-overlapping
2. Ambiguous
3. Degeneracy
4. Universal
165. Which of the following elements is a constituent of biotin?
1. Magnesium
2. Calcium
3. Phosphorus
4. Sulphur
166. Satellite RNA are present in some:
1. Viroids
2. Prions
3. Bacteriophages
4. Plant viruses
167. Which two distinct microbial processes are responsible for the release of fixed nitrogen as dinitrogen gas (N
2) to the atmosphere?
| 1. |
Aerobic nitrate oxidation, and nitrite reduction |
| 2. |
Decomposition of organic nitrogen, and conversion of dinitrogen to ammonium compounds |
| 3. |
Enteric fermentation in cattle, and nitrogen fixation by Rhizobium in root nodules of legumes |
| 4. |
Anaerobic ammonium oxidation, and denitrification |
168. Which of the following represents the action of insulin?
| 1. |
Increases blood glucose levels by stimulating glucagon production |
| 2. |
Decreases blood glucose levels by forming glycogen |
| 3. |
Increases blood glucose level by promoting cellular uptake of glucose |
| 4. |
Increases blood glucose levels by hydrolysis of glycogen |
169. Which one of the following is true for fungi?
1. They lack a rigid cell wall
2. They are heterotrophs
3. They lack nuclear membrane
4. They are phagotrophs
170. Why is a capsule advantageous to a bacterium?
| 1. |
It protects the bacterium from desiccation |
| 2. |
It provides means of locomotion |
| 3. |
It allows the bacterium to "hide" from the host's immune system. |
| 4. |
It allows the bacterium to attach to the surface |
171. The foetal ejection reflex in humans triggers the release of:
1. oxytocin from foetal pituitary
2. human Chorionic Gonadotropin (hCG) from placenta
3. human Placental Lactogen (hPL) from placenta
4. oxytocin from maternal pituitary
172. Which one of the following is a primary consumer in maize field ecosystem?
| 1. |
Grasshopper |
2. |
Wolf |
| 3. |
Phytoplankton |
4. |
Lion |
173. During the process of isolation of DNA, chilled ethanol is added to:
1. Precipitate DNA
2. Break open the cell to release DNA
3. Facilitate action of restriction enzymes
4. Remove proteins such as histones
174. When a man eats fish which feeds on zooplankton which has eaten small plants, the producer in the chain is:
| 1. |
Small plants |
2. |
Fish |
| 3. |
Man |
4. |
Zooplankton |
175. Syngamy can occur outside the body of the organism in:
1. Mosses
2. Algae
3. Ferns
4. Fungi
176. 
The figure gives an important concept in the genetic implication of DNA. Fill in the blanks A, B and C.
1. A - Maurice Wilkins B - transcription C - translation
2. A - James Watson B - replication C - extension
3. A - Erwin Chargaff B - translation C - replication
4. A - Francis Crick B - translation C - transcription
177. Uridine, present only in RNA is:
| 1. |
nucleoside |
2. |
nucleotide |
| 3. |
purine |
4. |
pyrimidine |
178. Which one of the following is one of the paths followed by air/O2 during respiration in an adult male Periplaneta americana as it enters the animal body?
| 1. |
Spiracle in metathorax, trachea, tracheoles, oxygen diffuses into cells |
| 2. |
Mouth, bronchial tube, trachea, oxygen enters cells |
| 3. |
Spiracles in prothorax, tracheoles, and trachea oxygen diffuses into cells |
| 4. |
Hypopharynx, mouth, pharynx, trachea, tissues |
179. Which of the following statements about enzymes is wrong?
| 1. |
Enzymes are denatured at high temperatures |
| 2. |
Enzymes are mostly proteins but some are lipids also |
| 3. |
Enzymes are highly specific. |
| 4. |
Enzymes require optimum pH and temperature for maximum activity. |
180. Which organization publishes the 'Red Data Book'?
| 1. |
IUCN |
2. |
UNEP |
| 3. |
WWF |
4. |
GEF |
*If above link doesn't work, please go to test link from where you got the pdf and fill OMR from there
CLICK HERE to get FREE ACCESS for 2 days of ANY NEETprep course








 is:
is:


















