Entropy change for vaporization of water if latent heat of vaporization for water is 540 cal/g (Given for = 0.51 K/molality) is
1. 21.66 cal/mole
2. 26.33 cal/mole
3. 46.32 cal/mole
4. 2.63 cal/mole
When 11.7 g of NaCl are dissolved in 200 g of water, the depression in freezing point is doubled than the depression caused by 342 g of cane sugar in 1000 g of water. From this information, what do you infer about the nature of the solute particles of NaCl in solution?
1. 92%
2. 72%
3. 100%
4. 1%
If boiling point of aqueous solution is , what is its freezing point? Given latent heat of fusion & vaporixation for water are 80 cal/g and 540 cal/g respectively.
1. -0.46
2. -0.36
3. 0.36
4. -0.56
Elevation in boiling point for 1 molal is three times of one molal aqueous solution of urea. Calculate the value of x, assuming 100% ionization of complex salt. solvent.
1. 5
2. 4
3. 1
4. 6
A maxima or minima obtained in the temperature, composition curve of a mixture of two liquids indicates:
1. an azeotropic mixture
2. a eutectic formation
3. that the liquids are immiscible with one another
4. that the liquids are partially miscible at hte maximum or minimum
The boiling point of an azeotropic mixture of water and ethyl alcohol is less than that of the theoretical value of a water and alcohol mixture. Hence, the mixture shows:
1. That solution is highly saturated
2. Positive deviation from Raoult's law
3. Negative deviation from Raoult's law
4. Nothing can be inferred
The substances whose solubility decreases with increase in temperature:
1.
2.
3.
4. all of these
Two solutions () containing and () containing are separated by semipermeable membrane as shown below. If on reaction with , produces blue colour of , the blue colour will be noticed in: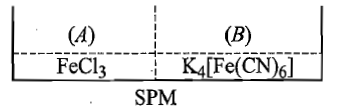
(1) (A)
(2) (B)
(3) in both (A) and (B)
(4) neither in (A) nor in (B)
Each pair forms ideal solution expect:
(1)
(2)
(3)
(4)
The value of for water is , calculated from glucose solution. The value of for water calculated for NaCl solution will be:
1. = 1.86
2. < 1.86
3. > 1.86
4. zero
Mole fraction of solute in an aqueous solution that boils at 100.104 is :
( for = 0.52 K )
1. 0.008
2. 0.004
3. 0.061
4. 0.996
At Abu mountains, water boils at 96. What amount of NaCl be added in 1 kg water so that it boils at 100. .
1. 225 g
2. 450 g
3. 200 g
4. 125 g
Freezing point of an aqueous solution is -0.166C. Elevation of boiling point of same solution would be-
(Kb = 0.512 K m-1 and Kf = 1.66 K m-1)
| 1. | 0.18°C | 2. | 0.05°C |
| 3. | 0.09°C | 4. | 0.23°C |
A substance is completely trimerized on dissolution in a solvent. The van;t Hoff factor (i) for such change is:
1. 1
2. 2
3. 3
4. 1/3
The amount of ice that will seprate out on cooling a solution containing 50 g of ethylene
glycol in 200 g water to -9.3 C is: (
1. 38.71 g
2. 38.71 mg
3. 42 g
4. 42 mg
The freezing point of aqueous solution that contains 5% by mass urea, 1.0% by mass KCl and 10% by mass of gulcose is: (kH=1.86 K molality)
1. 290.2 K
2. 285.5 K
3. 269.93 K
4. 250 K
The boiling point of an aqueous solution of a non-volatile solute is 100.15C. What is the freezing point of an aqueous solution obtained by diluting the above solution with an equal volume of water? The value of Kb and Kf for water are 0.512C and 1.86CK molality
:
(1)
(2)
(3)
(4)
The values of observed and calculated molar mass of silver nitrate are 92.64 and 170 respectively. The degree of dissociation of silver nitrate is:
1. 60%
2. 83.5%
3. 46.7%
4. 60.23%
A 0.001 molal solution of [Pt(NH)Cl] water had a freezing point depression of 0.0054C.If K f for water is 1.80 , the correct fromulation of the above molecule is:
1. [Pt(NH) Cl ]Cl
2. [Pt(NH) Cl]Cl
3. [Pt(NH) Cl]Cl3
4. [Pt(NH) Cl ]
The Henry's law constant for the solubility of N2 gas in water at 298 K is 1.0 atm. The mole fraction of N2 in air is 0.8. The number of moles of
N2 from air dissolved in 10 moles of water at 298 K and 5 atm pressure is:
(1) 4.010-4